
27 Jun Here’s why Emergent BioSolutions is worth investing!
Emergent BioSolutions, Inc. (NYSE: EBS), a life sciences company is developing a portfolio of vaccines and therapeutics for urgent health threats, announced the acceptance of a Biologics License Application (BLA) for AV7909 (Anthrax Vaccine Adsorbed, Adjuvanted) by the U.S. Food and Drug Administration (FDA). The Prescription Drug User Fee Act goal date for the candidate has been set for April 2023.
AV7909, an anthrax vaccine candidate, is intended for the treatment of post-exposure prophylaxis of disease following suspected or confirmed exposure to Bacillus anthracis in the age group of 18 through 65 years, administered in conjunction with recommended antibacterial drugs.
Kelly Warfield, senior vice president of research and development of the Company, commented,
“Over the last 20 years, Emergent has partnered with the U.S. government to lead this program from early- to advanced-stage development. As we progress toward licensure of AV7909, which is designed to follow a two-dose immunization schedule and elicit a faster immune response, we redouble our efforts to support the government’s overall preparedness and response strategy for large-scale emergencies involving anthrax and other threats to public health.”
The submission is based on the results from a Phase 3 clinical study of AV7909 that studied the immunogenicity and safety of the vaccine that follows a two-dose intramuscular administration schedule in healthy adults. The data from a Phase 2 study was also included, which studied the non-interference between AV7909 and antibacterial drugs approved for post-exposure prophylaxis of anthrax disease.
Besides drug development, the Company also offers contract development and manufacturing services in the form of Molecule-to-market services, initial R&D, and clinical studies through fill/finish and packaging.
Emergent BioSolutions, Inc. (NYSE: EBS)
Market Cap: $1.65B; Current Share Price: 32.76 USD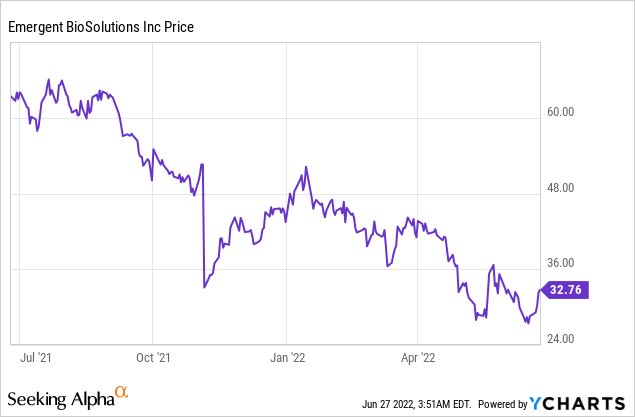
Data by YCharts
We take a holistic look at the Company through the SWOT analysis below:
Strength
Emergent has entered into a definitive agreement with Chimerix, Inc. to acquire exclusive worldwide rights to TEMBEXA® (brincidofovir), an FDA-approved antiviral intended to treat smallpox in adult and pediatric patients, including neonates. The acquisition helps add a small molecule therapeutically and is expected to be accretive with the anticipated product deliveries under the BARDA contract within three to six months from closing. The Company will pay Chimerix $225 million in one-time upfront payments in cash up to $100 million in milestone payments based on certain conditions.
The Company has 11 marketed products, including BioThrax® (Anthrax Vaccine Adsorbed), Anthrasil® [Anthrax Immune Globulin Intravenous (Human)], Raxibacumab injection, A fully human monoclonal antibody, VIGIV CNJ-016® [Vaccinia Immune Globulin Intravenous (Human)], BAT® [Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G) – (Equine)], RSDL® (Reactive Skin Decontamination Lotion Kit), and Trobigard®1 (Atropine sulfate/obidoxim ACAM2000® (Smallpox (Vaccinia) Vaccine, Live) as part of its government / medical countermeasures. The Company’s commercial portfolio consists of NARCAN® Nasal Spray (Naloxone HCl), Vaxchora® (Cholera Vaccine, Live, Oral), and Vivotif® (Typhoid Vaccine Live Oral Ty21a).
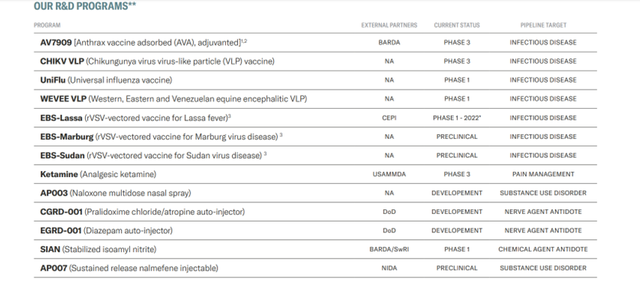
Image Source: Company
Emergent is developing an extensive pipeline of candidates consisting of CHIKV VLP (Chikungunya virus virus-like particle (VLP) vaccine) currently in Phase 3, UniFlu (Universal influenza vaccine) in Phase 1, WEVEE VLP (Western, Eastern, and Venezuelan equine encephalitic VLP) in Phase 1 EBS-Lassa (rVSV-vectored vaccine for Lassa fever) that will start Phase 1 trials in 2022, Ketamine (Analgesic ketamine) in Phase 3, besides multiple candidates in preclinical development.
In addition, the Company also has multiple platforms, such as The Emergard® auto-injector platform intended for rapid intramuscular delivery as part of emergency response medical treatments, hyperimmune specialty plasma product manufacturing platform, broad-spectrum antiviral iminosugars platform, and broad-spectrum antibiotics that target quinolone-resistant bacterial strains.
Emergent has eight manufacturing facilities that produce over 20 commercial products and has supported the development of more than 200 clinical candidates. In June 2012, the Company entered into a public-private partnership with Biomedical Advanced Research and Development Authority (BARDA) to establish a Center for Innovation in Advanced Development and Manufacturing (CIADM). BARDA has already awarded the Company’s CIADM four task orders to develop Ebola and Marburg therapeutics and a Zika vaccine.
Opportunity
Anthrax is caused by a spore-forming bacterium named Bacillus anthracis that usually affects animals. However, humans can get infected if they come in contact with the infected animal or inhale the spores. The spores cause a severe infection in animals and humans as they multiply rapidly upon entering the body and produce toxins. The spores are usually found in contaminated soil or water and are prevalent in agricultural regions like central and south America, Africa, Asia, and Europe. Treatment for the condition includes the administration of vaccines to prevent the disease and antibiotics in case of infection. However, inhaled anthrax is more challenging to treat and can prove to be fatal.
According to a report by Reports and Data, the global market for anthrax vaccines is likely to reach USD 863.7 million by 2026. The growth in the market will be driven by the increasing prevalence of the disease, improved immunization drives, government and regulatory support, and scientific advancements. Factors like lack of awareness, prohibitive treatment costs, and poor vaccine availability can hinder market development.
Weakness
In June 2022, Johnson & Johnson announced that it planned to terminate its COVID-19 vaccine supply deal with Emergent, citing a breach of the agreement. The companies had initially entered into a manufacturing agreement in 2020, and Emergent was to provide its vaccine development and manufacturing services for up to 5 years. Furthermore, the contract was valued at about $480 million for the first two years.
Both the companies are accusing each other of breach of contract. While J&J cited Emergent’s violations, including failure to supply Covid-19 vaccine drug substance, as the reason, Emergent has served a notice of material breach of an agreement to J&J. The Company states that J&J failed to provide forecasts for vaccine quantities and is not planning on purchasing the minimum quantity of product as per the initial agreement between the companies.
The Company was roped in to produce COVID-19 vaccines for AstraZeneca and Johnson & Johnson. However, on April 19, 2021, the Company filed with the United States Securities and Exchange Commission that it had halted production of any new material at its Bayview facility at the request of the FDA, which had initiated an inspection of the facility on April 12, 2021. The Company had to quarantine all existing material produced at the facility pending completion of the review and declaration of the findings.
The Company’s woes began in March 2021, when the news of workers at the Bayview facility mixing up the ingredients and ruining a batch of nearly 15 million vaccine doses of J&J became public. Fortunately, the mistake occurred during the early production stages, and none of the doses made it to distribution. This resulted in a temporary halt in Johnson & Johnson’s vaccine production at the facility. The Company stated that it was stepping up to implement tighter controls and would ensure stringent quality checks and test runs to adhere to its high-quality standards.
Johnson and Johnson took over the responsibility of the manufacturing facility, while AstraZeneca moved the production of its viral vector product to another location.
The Company also received a damning FORM 483 from the FDA, which lists unsanitary conditions, inadequate facility size, substandard employee training, and insufficient written procedures as some of the issues with its Bayview facility. Emergent had to issue a press release stating that it is working closely with the FDA and Johnson & Johnson to address the problems as quickly as possible. However, the incidents severely damaged the Company’s chances of landing more manufacturing contracts in the near term due to these events.
Threats
The Company’s pipeline has multiple candidates being evaluated in diverse indications. The other symptoms that the candidate is being assessed are either in Phase 2 / 3 trial stage or undergoing preclinical development. Clinical Trials are fraught with risk and uncertainty. There is a possibility that the candidates in the Company’s developmental pipeline may not be able to meet their clinical endpoints in trials.
The Company may fail to receive regulatory approval for any other candidates, resulting in a setback for the other candidates in the pipeline.
However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to meet endpoints in any of its ongoing trials. The success of its clinical trials will allow the Company to advance its pipeline, but it should also be prepared to face any setbacks in case its ongoing attempts fail to meet its endpoints.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.emergentbiosolutions.com/wp-content/uploads/2022/03/EBSCorporateFactSheet-031822-001.pdf





No Comments