
29 Nov 5 Reasons Why Rafael Holdings Is a Risky Investment!
“Realignment” of Management Team
Rafael Holdings, Inc. (NYSE: RFL), announced the realignment of its management team, including the departure of the CEO Ameet Mallik, William Conkling, chief commercial and business officer and Ashok David Marin, chief legal officer among others. The responsibilities of the CEO will now be taken over by Chairman Howard Jonas as of February 1, 2022, with Mr.Mallik continuing to support the company by being a member of the board and heading the transition committee.
Ameet Mallik, outgoing CEO and continuing member of the Board, commented,
“I joined Rafael Holdings to recruit a top-tier leadership team to evolve the company into a fully integrated commercial organization. While the recent results of Rafael Pharmaceuticals’ two Phase 3 clinical trials for CPI-613® (devimistat) in metastatic pancreatic cancer and relapsed or refractory acute myeloid leukemia are disappointing, I am proud of and grateful for the team’s significant contributions. We are fortunate to have an early-stage pipeline backed by world class scientific advisors, which we believe has great potential.”
The new management team will see Patrick Fabbio taking over the role of the president, in addition to continuing as Chief Financial Officer, while Dr. Mimi Huizinga will act as both Chief Medical Officer and Head of Research and Development.
The move comes on the heels of the Company’s subsidiary Rafael Pharmaceuticals announcing that its lead candidate devimistat (CPI-613) failed to achieve its primary objective of overall survival (OS) in a crucial Phase III trial. Furthermore, another late-stage trial ARMADA2000, evaluating the candidate in patients with relapsed or refractory acute myeloid leukemia (AML), was halted due to lack of efficacy based on recommendation of the data monitoring committee.
Rafael Holdings, Inc. (NYSE: RFL)
Market Cap: $122.13M; Current Share Price: 5.90 USD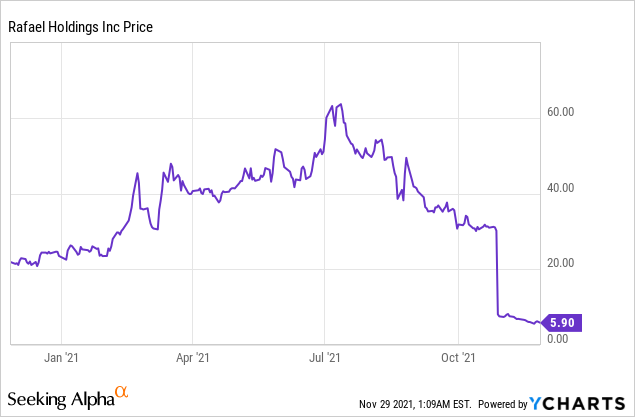
Data by YCharts
Setbacks in Phase 3 Clinical Trials
Rafael Holdings has interests in two clinical-stage oncology companies Rafael Pharmaceuticals, Inc. and LipoMedix Pharmaceuticals Ltd and a cancer drug development initiative namely the Barer Institute.
The Company is developing CPI-613® (devimistat), a first-in-class compound that targets enzymes present in the mitochondria that play an important role in cancer cell metabolism. The candidate is currently being evaluated in multiple clinical trials as a single agent or in combination with standard therapies in rare solid tumors or blood cancers.
CPI-613® (devimistat), has received an orphan drug designation from the U.S. Food and Drug Administration (FDA) for indications such as pancreatic cancer, acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), peripheral T-cell lymphoma, Burkitt’s lymphoma and soft tissue sarcoma and biliary tract cancer. The European Medicines Agency (EMA) has granted the candidate orphan drug designation for the treatment of pancreatic cancer, acute myeloid leukemia (AML) and Burkitt’s lymphoma.
The results from a Phase 3 clinical trial, AVENGER 500 that was evaluating CPI-613® (devimistat) in combination with modified FOLFIRINOX (mFFX) as a first-line therapy in patients with metastatic adenocarcinoma of the pancreas, showed that the candidate failed to meet its primary endpoint of overall survival. The trial had randomized 528 patients to receive either devimistat in combination with modified FOLFIRINOX (mFFX) or FOLFIRINOX. The results show that Devimistat given with mFFX failed to significantly improve overall survival (HR=0.95, p=0.66). The median overall survival in the devimistat and mFFX arm was 11.1 months, compared to 11.7 months in the FOLFIRINOX arm.
An early-stage pipeline
In November 2021, the Company’s lead candidate CPI-613® (devimistat) was granted an orphan drug designation by the European Medicines Agency (EMA) for the treatment of Burkitt’s lymphoma, a rare form of cancer, which accounts for 1% of adult lymphoma and up to 30% of childhood NHL. Moreover, the Company enrolled of the first patient in the APOLLO 613 Phase 1/2 clinical trial of CPI-613® (devimistat) in combination with hydroxychloroquine in patients with clear cell sarcoma.

Image Source: Company
The candidate is being evaluated in multiple trials including a Phase II in relapsed or refractory Burkitt’s Lymphoma/ Leukemia or High-Grade B-cell Lymphoma, Relapsed or Refractory Clear Cell Sarcoma and as a first-line locally advanced, unresectable or metastatic biliary tract cancer. Devimistat, in combination with gemcitabine + nab-paclitaxel is undergoing a Phase 1 trial in first-line metastatic pancreatic cancer and in combination with FOLFOXIRI+ bevacizumab in metastatic colorectal cancer. However, these are all at an early-stage of development and will require considerable time and resources for development.
A Missed Opportunity
The pancreas, approximately six inches long and located behind the stomach in the abdomen, plays a crucial role in regulation of blood sugar as well as digestion. They secrete enzymes such as insulin and digestive juices that assist with the exocrine and endocrine functions of the body. However, the cells in pancreas may develop mutations in their DNA, leading to their uncontrolled growth and formation of tumors. This form of cancer most commonly develops in the cells lining the ducts of the pancreas and is named pancreatic adenocarcinoma or pancreatic exocrine cancer. Less frequently it can even develop in the neuroendocrine cells of the pancreas, leading to pancreatic neuroendocrine tumors.
The American Cancer Society estimates that nearly 57,600 people will be diagnosed with pancreatic cancer in 2020, while 47,050 people will succumb to the disease. In addition, it accounts for 3 percent of cancers in the US alone and about 7 percent of all cancer deaths.
The definite cause of pancreatic cancer is still unknown, however smoking, diabetes, pancreatitis, obesity, presence of BRCA2 gene mutation, family Lynch syndrome and familial atypical mole-malignant melanoma (FAMMM) syndrome are some of the contributing factors. In most cases the symptoms do not manifest early on, until the disease advances and spreads to other organs as well. However unexplained weight loss, Jaundice, irregular bowel movements are some of the indicators.
Screening and diagnosis use tools such as Endoscopic Ultrasound, Magnetic Resonance Imaging (MRI) Positron Emission Tomography (PET) and Computerized Tomography (CT) scans, besides conducting a biopsy and blood test. The current treatment options are surgery, chemotherapy, radiation and palliative care for more advanced cases.
According to a report by Grand View Research Inc, the pancreatic treatment market is expected to reach USD 4.2 billion in 2025. A growing geriatric population, lifestyle-related diseases and increasing awareness and availability of treatment options will drive the growth in the market.
A Risky One Candidate Pipeline
The Company’s pipeline consists of only one candidate being evaluated in multiple indications. Clinical Trials are fraught with risk and uncertainty. There is a possibility that the candidates in the Company’s developmental pipeline may not be able to meet their clinical endpoints in trials. However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to meet endpoints in any of its ongoing trials. The success of its clinical trials will help the Company advance its pipeline but it should also be prepared to face any setbacks, in case its ongoing trials fail to meet their endpoints.
Key Takeaways
In December 2020, The Barer institute has entered into an agreement with Princeton University’s Office of Technology Licensing for technology from the laboratory of Professor Joshua Rabinowitz, for exclusive worldwide license to its SHMT (serine hydroxymethyltransferase) inhibitor program and related intellectual property. The tie-up has the potential to introduce more candidates into the pipeline in the future.
The Company has built a strong intellectual property rights portfolio with protection until 2028 across U.S., Canada, EU, Israel, Australia and Asia.
In June 2019, the Company entered into a licensing agreement with Ono Pharmaceutical Co., Ltd. for exclusive development and commercialization rights to devimistat and other related compounds in Japan, South Korea, Taiwan and ASEAN countries. Rafael received a one-time upfront payment of $12.9 million and up to an additional $150.3 million in development and commercial milestones.
Rafael Holdings had earlier announced a strategic merger with Rafael Pharmaceuticals, which will be up for shareholder vote and the process is likely to be completed in 2022. The move is to “create a publicly traded, late-stage clinical oncology company positioned to synergistically leverage the assets of both companies”.
The Company had $6.2 million in cash at the end of Q3,2021 and will need to raise more capital to advance its pipeline and fund its operations. Though the Company has some real estate properties, which currently generate around $1 million a quarter, it would need more funds to continue its clinical development and operations.
The failure of the candidate in one trial does not mean it will fail in all its trials, however considering devimistat is being targeted as a combination therapy, it would take a while for the Company to figure out the best combination that works. It is safe to say that it would be advisable to watch the Company’s next few steps from the sidelines.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://finance.yahoo.com/news/rafael-holdings-realigns-leadership-tem-120000144.html
https://www.mayoclinic.org/diseases-conditions/pancreatic-cancer/symptoms-causes/syc-20355421
https://rafaelpharma.com/wp-content/uploads/Rafael-Pharma_CPI-613%C2%AE-devimistat_Fact-Sheet.pdf





No Comments