
05 May Upcoming Catalysts Make Tonix Pharmaceuticals a Must Watch!
The Company is developing a pipeline of candidates focused on small molecules and biologics that can treat central nervous system (CNS) and immunology disorders. Tonix Pharmaceuticals Holding Corp (NASDAQ: TNXP) lead candidate for CNS is TNX-102 SL (sublingual formulation of cyclobenzaprine), a non-opioid, centrally-acting analgesic that is currently in mid-Phase 3 development for the treatment of fibromyalgia. The Company reported positive data from a Phase 3 Relief study that met its pre-specified primary and secondary endpoints of pain reduction, improved sleep, reducing fatigue and overall improvement in symptoms and functions.
TNX-102 SL primarily targets mechanisms associated with sleep disturbances, one of the most common symptoms of fibromyalgia, which is believed to improve the perception of pain and the body’s ability to modulate pain effectively. The product is a sublingual drug candidate (under the tongue), which has been designed to address the limitations of oral formulations of cyclobenzaprine by offering improved delivery and absorption. The drug has shown to have high affinity to Serotonin-2A (5-HT2A) receptor, Alpha-1-adrenergic receptor and Histamine-1 receptor and works as an antagonist at all three sites. Most importantly it is designed for long-term use.
Tonix has randomized 50 percent of the participants in the second pivotal phase 3 RALLY study in fibromyalgia, which will be enrolling approximately 670 participants. The Company intends to announce the results from an interim analysis in Q3,2021, followed by topline results in Q4,2021. The Company intends to submit a New Drug Application for the candidate to the U.S. Food and Drug Administration (FDA) in 2022, based on the outcome of its second phase 3 study.
Tonix Pharmaceuticals Holding Corp (NASDAQ: TNXP)
Market Cap: $ 375.49M; Current Share Price: 1.15 USD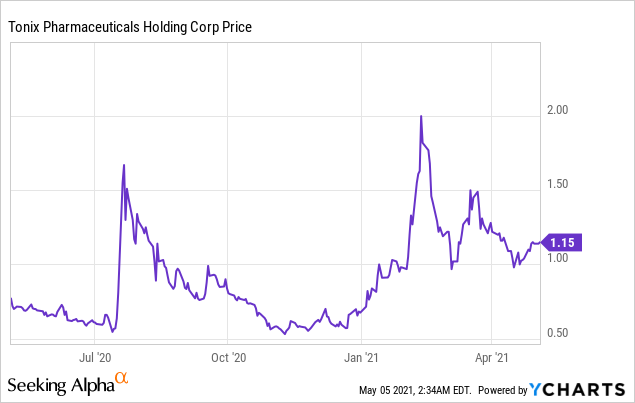
Data by YCharts
Industry
Fibromyalgia is widespread musculoskeletal pain that is accompanied by fatigue, emotional and mental stress and sleep issues. The disorder is believed to be caused by abnormal pain perception processing that amplifies the sensation of pain, by impacting the processing of pain signals by the brain and the spinal cord.
Most common symptoms of Fibromyalgia include muscle pain, stiffness, numbness or tingling in the hands and feet, fatigue, issues with concentration and memory, depression and anxiety to name a few. The risk factors include age, gender or co-morbidities such as Lupus or Rheumatoid Arthritis, obesity, family history and muscle injury or post-traumatic stress disorder (PTSD). Currently there is no cure for the condition, however the symptoms can be managed through medication and physical therapy such as prescription drugs and over-the-counter pain relievers, muscle strengthening exercises and Cognitive behavioral therapy (CBT).
According to a report by Fortune Business Insights, the global fibromyalgia treatment market is poised to reach 1.41 billion by 2021, growing at a CAGR of 9.2 percent, from 764.1 million in 2020. Increased research and development and a large pipeline of drugs are fueling the growth in the market. Fibromyalgia affects 2 to 4% of the population, or nearly 6 to 12 million people in the U.S alone, and is 2 to 7 times more common in women than men as per estimates.
Company
Tonix is also awaiting the clearance for its Investigational New Drug (IND) from FDA for initiating a phase I clinical trial of TNX-1800, a single-administration COVID-19 vaccine candidate that elicits T cell immunity and is based on its horsepox virus vector, TNX-801. The candidate has demonstrated strong immune response to SARS-CoV-2 in non-human primates at low-doses, and the Company intends to announce results from an efficacy study of TNX-1800 in vaccinated non-human primates, that have been challenged with live SARS-CoV-2 in Q1,2021. In April 2021, the Company entered into an exclusive worldwide agreement with OyaGen to develop SARS-CoV-2 inhibitor TNX-3500 (sangivamycin) for the treatment of COVID-19 and other potential future viral diseases. The candidate’s active ingredient has previously demonstrated safety and tolerability in humans in cancer studies at the U.S. National Cancer Institute. The Company has received licenses for technology and patents associated with TNX-3500, in addition to licenses for other compounds related to TNX-3500 as well.
Seth Lederman, M.D President and Chief Executive Officer (CEO) of TONIX, commented,
“We are excited to expand our pipeline and we look forward to developing TNX-3500 as a potential treatment for COVID-19 and emerging variants. TNX-3500 is in the pre-Investigational New Drug (IND) phase of development with encouraging early data from cell culture infectivity studies with SARS-CoV-2. We believe that its potency on SARS-CoV-2 inhibition in tissue culture and its tolerability in humans from prior studies suggests that TNX-3500 may qualify for expedited clinical development.”
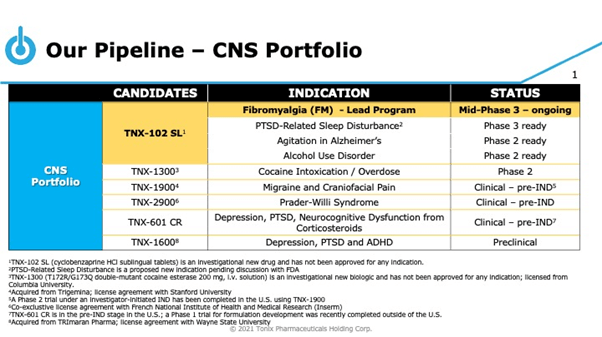
Image Source: Company
Tonix is also gearing up to file an IND and initiate clinical trials for TNX-2100, a diagnostic skin test for measuring exposure to SARS-CoV-2 as well as related T cell immunity in Q2, 2021. In February 2021, the Company received a pre-IND meeting written response from the FDA with guidance for the development of the candidate. Furthermore, the Company is planning an IND application for TNX-1900 (intranasal potentiated oxytocin) in Q2,2021, besides initiating a Phase 2 study of TNX-1900 for the prophylactic treatment of chronic migraine in Q3,2021.
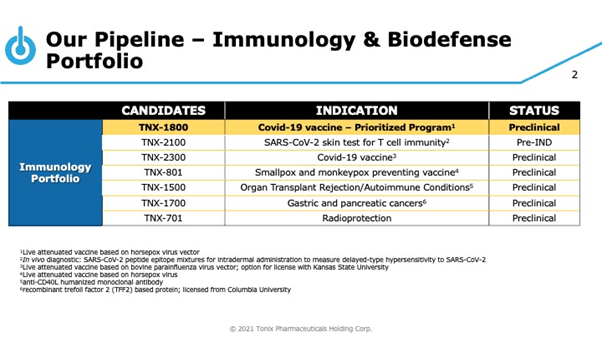
Image Source: Company
The Company has an extensive and diverse pipeline of candidates covering areas with high unmet medical needs such as Agitation in Alzheimer’s, Cocaine Addiction / Overdose, Migraine and Craniofacial pain, Prader- Willi Syndrome, Depression, PTDS and ADHD as part of its CNS development pipeline. Tonix’s Immunology Portfolio consists of vaccines against smallpox and monkeypox, organ transplant rejection and autoimmune conditions, gastric and pancreatic cancers and radioprotection.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.cdc.gov/arthritis/basics/fibromyalgia.htm
https://www.fortunebusinessinsights.com/fibromyalgia-treatment-market-105016
https://www.tonixpharma.com/pipeline/tnx-102-sl-for-fibromyalgia





No Comments