
24 Jan 7 Reasons to Put Adicet Bio on your Watchlist!
Adicet Bio, Inc. (NASDAQ: ACET) is a biotechnology Company working on bringing allogeneic gamma delta T cell therapies for cancer and other therapies. The Company had merged with resTORbio, Inc. to create a combined entity, in September 2020. We take a look at the Company’s technology and pipeline in the article below:
The Science
Adicet is a clinical-stage biotechnology Company that is focussed on developing off-the-shelf T cell therapy for the treatment of cancer. The Company seeks to leverage the power of gamma delta T cells to create universal immune cell therapy.
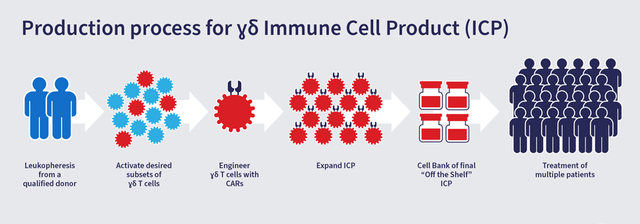
Image Source: Company
The Company’s approach is an improvement over existing engineered autologous alpha beta T cell products that are yet to prove their efficacy in solid tumors. The technology being developed by the Company, aims to use the tumor specific immunity of gamma delta T cells, which it believes has superior potential, when compared to alpha beta T cells in both hematological cancers and solid tumors.
Delta Cells combine the features of adaptive and innate immunity to target and eliminate tumor cells, with least peripheral cell damage. One of the most prominent advantages of using gamma delta T cells is that unlike alpha beta T cells, they carry out the tumor killing function in an MHC (major histocompatibility complex) independent manner, thereby eliminating the risk of Graft vs Host Disease (GvHD). They are also capable of naturally homing to tissues and have demonstrated cytotoxicity and anti-tumor activity in vitro and in vivo in mouse models. Adicet’s proprietary technology enables it to activate and expand numerous subsets of gamma delta T Cells to sufficient numbers for use in clinical settings.
Adicet is using chimeric antigen receptors (CARs) and T cell receptors (TCRs) to engineer gamma delta T cells that can be applied for the treatment of numerous hematological and solid tumor cancers, and other diseases. The Company’s proprietary T cell receptor-like antibody (TCRL) platform technology can generate CAR’s with the potential to recognize intracellular proteins and can be used to arm gamma delta T cells, act as T cell engager (bispecific) or as Antibody Drug Conjugates (ADC).
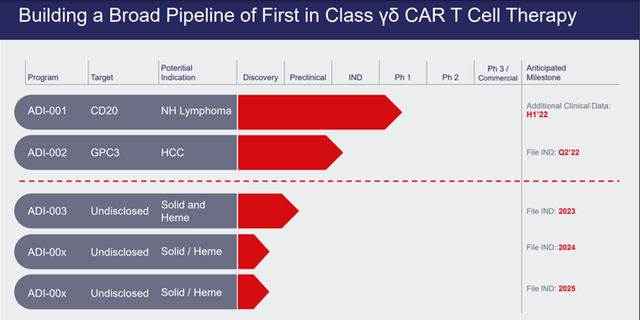
Image Source: Company
Positive Interim Results from ADI-001
The Company’s pipeline consists of ADI -001 intended for the treatment of Non-Hodgkins Lymphoma, which demonstrated complete and near complete responses starting at lowest dose level in Phase 1 study (ORR=75%, CR=50%) in a dose escalation Phase 1 study evaluating the safety and tolerability of the candidate. The study had enrolled 6 patients, out of which two patients were unable to reach the 28 day assessment and hence were not evaluated for efficacy as per protocol. Out of the remaining four, two achieved complete response and one achieved partial response.
Patients in the study were pre-treated and had received a median of five lines of prior systemic therapy. The other candidates in the Company’s pipeline are ADC-002, for the treatment of HCC, which is currently undergoing preclinical studies, ADI-00x in Solid Tumors and ADI-00x in Solid and Haematological cancers.
Adicet Bio, Inc. (NASDAQ: ACET)
Market Cap: $466.98M; Current Share Price: 11.93 USD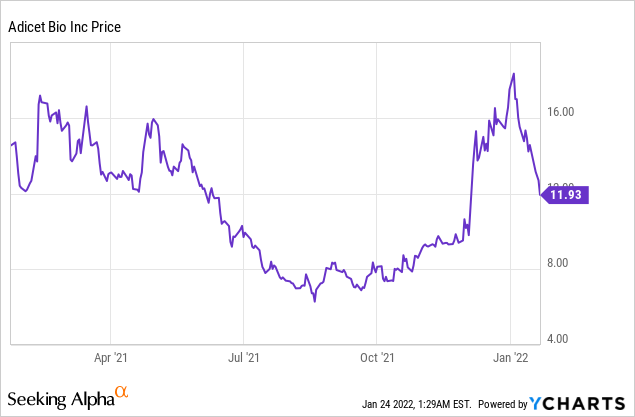
Data by YCharts
Upcoming Milestones
The Company is likely to announce additional data from the Phase 1 NHL study in the first half of 2022. Furthermore, Adicet is working on identifying RP2D (recommended Ph2 dose) for the candidate.
The Company intends to conduct an expansion study in patients relapsing after autologous CD19 CAR T, along with an expansion study in NHL subtypes (DLBCL, MCL, FL).
Adicet intends to file for an Investigational New Drug (IND) application for ADI-002 in GPC3+ tumors and initiate a Phase 1 in HCC, squamous cell carcinoma of the lung, and other GPC3+ tumors.
Strong Intellectual Property Rights Portfolio
Adicet has built a robust intellectual property rights portfolio that cover various aspects of its platform such as γδ T cell Expansion γδ T cell Optimized Constructs, Novel Targeting Ligand Platform encompassing methods of treatment, composition and methods of expansion / treatment and multiple pending patents.
In addition, the IP also covers TCR-like Antibodies such as carcinoma target and melanoma and glioblastoma Target, along with multiple pending patent applications covering compositions and methods of treatment.
Strategic Collaboration with Regeneron
The Company has entered into a collaboration and licensing agreement with Regeneron to develop next-generation engineered immune cell therapeutics, with an aim to develop immune cells with fully human chimeric antigen receptors (CARs) and T-cell receptors (TCRs).
The agreement seeks to generate multiple clinical product candidates in hematological and solid tumor cancers. Adicet received $25 million upfront payment as well as research funding over a five-year research term. The Company can use Regeneron’s proprietary mice and has the right to develop and commercialize ADI-001, their first collaboration target.
Regeneron (NASDAQ: REGN), has an option to exercise exclusive rights for ADI-002 and other additional targets. In case Regeneron exercises its option, the Company is eligible to receive an option exercise fee and has the right to participate in the funding, promotion and profit-sharing of the product or receive royalties.
In September 2021, the Company entered into a collaboration with Twist Bioscience Corporation (NASDAQ: TWST) to develop gamma delta T cell therapies against five undisclosed targets. The companies will combine Twist’s proprietary single chain fragment variable (scFv) and VHH (nanobody) technologies with Adicet’s engineering and discovery of unique CARs to generate novel gamma delta CAR T cell products. Twist is eligible to receive an upfront technology license fee, in addition to clinical and regulatory milestones and royalties.
Significant Market Opportunity
Lymphoma, a common form of blood cancer occurs when lymphocytes, a type of white blood cell, grow and multiply uncontrollably and manifest as Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). These in turn cause tumors by spreading to other parts of the body such as lymph nodes, spleen and bone marrow.
Lymphocytes are mainly categorized into B lymphocytes (B cells) and T lymphocytes (T cells) with the indolent (slow-growing) B lymphocytes (B cells) leading to Marginal zone lymphomas (MZLs), this accounts for an estimated eight percent of all non-Hodgkin lymphoma (NHL) cases. NHL can be further categorised into chronic lymphocytic leukemia, cutaneous B-cell lymphoma, Cutaneous T-Cell lymphoma. Follicular lymphoma and waldenstorm macroglobulinemia.
The disease manifests in the form of lymph nodes swelling, abdominal pain, chronic fatigue, unexplained weight loss to name a few. The risk factors include use of immunosuppressive medication, HIV/ Epstein-Barr infection/ Helicobacter pylori and old-age.
Image Source: Imbruvica
Globally the non-Hodgkin lymphoma (NHL) market is likely to reach over $9.2 billion by 2020, according to the pharmaletter. NHL accounts for 4% of all cancers in the United States and affected nearly 81,560 people (45,630 men and 35,930 women) in the United States in 2021 according to an estimate.
Financial Results
As per its Q3, 2021 financial results, the Company had cash, cash equivalents and marketable debt securities of $192.2 million as of September 30, 2021, as against $94.6 million as of December 31, 2020.
In December 2021, the Company completed a $100-million follow-on public offering of 7,187,500 shares of its common stock at a price of $14.00 per share. This included exercise in full by the underwriters of their option to purchase up to an additional 937,500 shares of common stock, at a public offering price of $14.00 per share, less underwriting discounts and commissions.
Conclusion
Adicet is one of the first companies to demonstrate clinical activity for an off-the-shelf gamma delta CAR-T cell therapy. The candidate has the potential to maintain or improve the response rate and is yet to show dose-limiting toxicities or other serious adverse events in the form of neurotoxicity syndrome or cytokine release syndrome. The Company’s positive results for ADI-001 are based on a trial that had 6 patients enrolled. The expansion studies will have to replicate the results from this trial to be able to further its clinical development.
Clinical-Stage companies offer an exciting investment opportunity with massive upside potential. Most of these companies bring new and highly differentiated approaches, advanced scientific knowledge and a zeal for innovation to the table.
However, a word of caution is in order as clinical trials are fraught with risk and uncertainty. Even the slightest setback can prove detrimental to the existence of these companies. Failure to meet clinical endpoints, lack of funding or rejection from regulatory authorities are risks that these companies have to bear in pursuit of excellence.
The Company is years away from an actual product or generating any significant revenue and hence a very risky investment like most biotechnology companies that are at the initial stages of development.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.adicetbio.com/science/#tcell
https://www.cancer.net/cancer-types/lymphoma-non-hodgkin/statistics
https://www.thepharmaletter.com/article/non-hodgkin-lymphoma-market-to-grow-to-9-2-billion





No Comments