
02 Jul All Eyes on Tarsus Pharmaceuticals!
Tarsus Pharmaceuticals, Inc. (NASDAQ: TARS), a clinical-stage pharmaceutical company targeting ophthalmic conditions with large unmet medical needs, recently announced that the pivotal Phase 2b/3 Saturn-1 trial which is evaluating TP-03 (lotilaner ophthalmic solution, 0.25%), in patients with Demodex blepharitis has met all pre-specified primary and secondary endpoints.
TP-03, is the Company’s lead candidate that inhibits parasite-specific GABA-Cl channels, to paralyze and eliminate mites and other parasites, and has till date been evaluated in four phase 2 trials successfully meeting endpoints in all of them. The candidate has been well-tolerated with no severe adverse events reported or leading to discontinuation across all the trials.
The results from the study show that the candidate showed
- Statistically significant complete collarette cure at day 43 in patients with Demodex blepharitis treated with TP-03 compared to vehicle, which was the primary endpoint and
- mite eradication at day 43 and composite cure based on complete collarette and erythema cures at day 43, the secondary endpoint.
In addition, TP-03, also demonstrated significant improvements within two weeks on multiple endpoints.
Bobak Azamian, M.D., Ph.D., President and Chief Executive Officer of Tarsus, commented,
“We believe the results from our Saturn-1 trial mark an important moment in Demodex blepharitis research, showing the potential of TP-03 to target the underlying cause of this disease and potentially become the standard of care for patients and clinicians. We expect to provide topline results for our second pivotal trial for TP-03, Saturn-2, in Q1 of 2022. If Saturn-2 trial data is positive, similar to the positive Saturn-1 results, we expect both Saturn-1 and Saturn-2 trials to support our submission of a New Drug Application (NDA) for TP-03 for the treatment of Demodex blepharitis in 2022.”
Saturn-1 was a randomized controlled, multicenter, double-masked trial, with 421 adults aged 18 and over, having more than 10 collarettes on the upper lid and at least mild erythema of the upper eyelid margin. The drug was administered twice per day in each eye for six weeks.
Tarsus is engaged in a pivotal Saturn-2 (Phase 3) trial, which is evaluating TP-03 on the same endpoints as Saturn-1. The enrollment of the study began in May 2021 and the Company expects to announce the topline results from the Saturn-2 trial in Q1 2022. Based on the results, the Company intends to submit a New Drug Application (NDA) to the FDA in 2022.
Tarsus Pharmaceuticals, Inc. (NASDAQ: TARS)
Market Cap: $595.21M; Current Share Price: 29.00 USD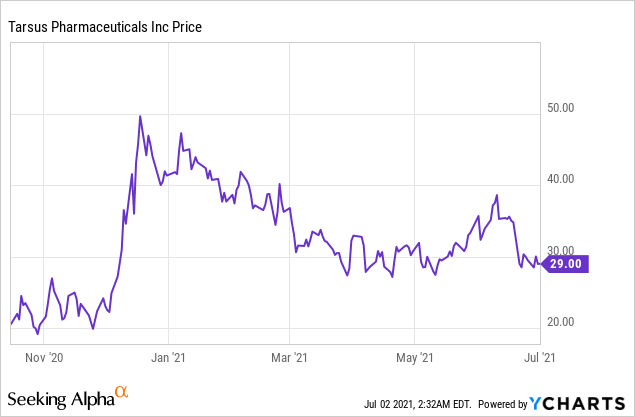
Data by YCharts
Industry
Blepharitis is an inflammation in the eyelid, characterized by red, swollen lids and formation of a crust on the eyelids. The condition is chronic in nature and can be difficult to treat causing pain and discomfort, severely affecting the quality of life of those affected. Blepharitis isn’t contagious or a life-threatening condition and is usually categorized into Anterior blepharitis and Posterior blepharitis.
A survey conducted by The Ocular Surface (2009) of U.S ophthalmologists in the U.S, revealed that between 37% and 47% of patients had some degree of blepharitis. The primary cause of blepharitis is Demodex Mites in the eyelid, which feed on dead skin cells. Other causes can include dry eyes, bacterial or fungal infection, dysfunction of the meibomian gland and Seborrheic dermatitis among others. The condition manifests in the form of redness in the eyes, formation of yellow debris in the base of the eyelashes, blurry vision, itchy eyes, watery eyes to name a few.
Most common forms of treatment include use of water compress, eye drops, antibiotics, corticosteroid eye drop or ointment, in severe cases doctors may recommend eyelid margin cleaning, thermal pulsation and intense pulsed light (IPL).
The primary cause of blepharitis is attributed to demodex mites, which are mostly found in the hair follicles, eyelashes etc., These mites mostly feed on dead skin and leave behind waste products in the eye glands. They are actually beneficial in limited numbers, but the problem occurs when they reproduce uncontrollably and lead to severe damage to the skin and eyes, including eczema and other such conditions.
According to estimates, the condition affects more than 25 million American, out of which 45% of blepharitis, or about 9 million cases are caused by the Demodex mites. Currently there are no approved treatments for the condition and most of the efforts are directed towards alleviating the symptoms.
Company
The Company is focused on addressing the unmet medical needs by leveraging cutting-edge technology to create novel therapeutics for treatment of highly prevalent diseases, with the initial focus being eye care. Tarsus aims to address the DEMODEX BLEPHARITIS, a condition that affects close to 20 million Americans and believes that the number of patients may be much higher than reported.
Tarsus has conducted studies that show that 58 percent of the people who visit an ophthalmologist at an eye care clinic exhibit signs of demodex infestation and estimates that the number of patient infections may be close to 25 million in the U.S alone. A majority of these patients experience symptoms for more than four years and face challenges in simple tasks such as wearing makeup, driving at night and worry about the appearance of their eyes or eyelids.
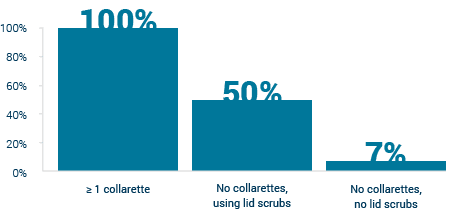
Image Source: Company
Tarsus is developing lotilaner, which has the potential to become the first FDA-approved treatment for Demodex blepharitis. In addition, TP-03 is also being evaluated for the treatment of Meibomian gland disease (MGD). The Company will be testing a preservative-free formulation of TP-03, after the completion of its NDA submission and has entered into a partnership with LianBio for development of Demodex blepharitis and MGD in China.
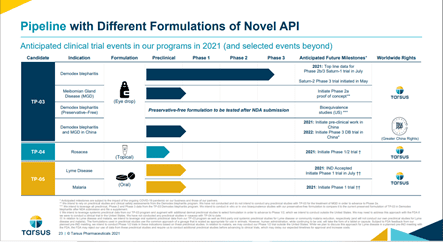
Image Source: Company
Furthermore, Taurus is currently conducting preclinical studies for the development of TP-04, intended for the treatment of Rosacea. The Company’s pipeline also consists of TP-05 for the treatment of Lyme Disease and Malaria.
Tarsus is currently engaged in a phase 2a proof of concept in MGD and a Phase 1/2 trials in rosacea, Lyme disease and malaria.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://tarsusrx.com/pipeline/
https://ir.tarsusrx.com/static-files/a4f36edb-9d92-46a2-a8c4-49a3efd6ea94





No Comments