
30 Jun ANAVEX®2-73: A Potential Blockbuster Drug in the Making!
Anavex Life Sciences Corp (NASDAQ: AVXL) is a clinical-stage biopharmaceutical company that is developing a pipeline of therapeutic candidates that target the Central Nervous System (CNS) by leveraging its expertise in precision genetic medicine. The Company has announced that its predictive biomarker of response established with SIGMAR1 mRNA expression, correlates significantly with responses in primary and secondary clinical efficacy endpoints, from a Phase 2 trial with 132 patients with Parkinson’s disease dementia.
ANAVEX®2-73 works by activating the sigma-1 receptor (SIGMAR1), which results in the restoration of complete housekeeping function within the body and plays a critical role in restoring neural cell homeostasis and promoting neuroplasticity. This is the first time that a drug-specific biomarker correlates with clinical efficacy endpoints in Parkinson’s Disease.
The treatment with ANAVEX®2-73 demonstrated significant (p = 0.035) mRNA expression increase of SIGMAR1,and correlation with clinical efficacy as measured by primary cognitive efficacy endpoints, CDR system Continuity of Attention (CoA) (p = 0.029) and CDR system Power of Attention (PoA) (p = 0.015), and secondary Parkinson’s efficacy endpoints Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)[4], MDS-UPDRS Part III (p = 0.024) and MDS-UPDRS Total (p = 0.038).
In addition, the candidate also showed statistically significant improvements compared to placebo (ITT population) for MDS-UPDRS Total score (p = 0.034). From baseline to end of trial at 14 weeks, MDS-UPDRS Total score improved by -10.98 points in the ANAVEX®2-73 high dose group and worsened by 3.53 points in the placebo group, an adjusted mean difference of -14.51 points (p = 0.034). This corresponds to a relative improvement of 18.9 % over 14 weeks.
Most importantly, ANAVEX®2-73 has shown to slow the progression of motor and non-motor symptoms in moderately advanced patients with Parkinson’s.
Christopher U. Missling, PhD, President & Chief Executive Officer of Anavex commented,
“We believe that the easily accessible predictive biomarker combined with the observed efficacy is a consistent explanation of the efficacy in this second largest CNS indication with unmet medical need. This data further strengthens the foundation of ANAVEX®2-73 as a cross-platform CNS drug.”
The Company was recently awarded a research grant by the Michael J. Fox Foundation (MJFF) for an imaging-focused Parkinson’s disease clinical trial with ANAVEX®2-73. Anavex intends to submit this data to the FDA for regulatory guidance.
Anavex Life Sciences Corp (NASDAQ: AVXL)
Market Cap: $1.71B; Current Share Price: 23.63 USD 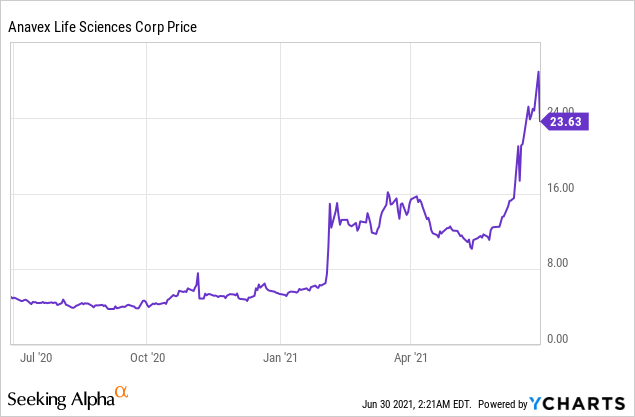
Data by YCharts
Strength
The Company’s lead candidate ANAVEX®2-73 has demonstrated improved Mini Mental State Examination (MMSE) and Alzheimer’s Disease Cooperative Study Group – Activities of Daily Living Inventory (ADCS-ADL) scores through 148 weeks in a Phase 2a clinical study. In addition, the Company has been able to identify novel genomic biomarkers such as the precision medicine biomarker, SIGMAR1 gene expression, which has led to them being applied to a Phase 2b/3 Alzheimer’s disease (AD) stud, along with other potential indications including Parkinson’s Disease Dementia (PDD) and Rett Syndrome (RTT).

Image Source: Company
In June 2021, the Company announced that it has exceeded the target of enrollment for the ANAVEX®2-73 (blarcamesine) Phase 2b/3 study in Alzheimer’s disease, the topline results from which are expected to be announced in mid-2022. The study will be using the SIGMAR1 gene expression, which has shown significant clinical benefit in cognition and activities of daily living and function, in an earlier Phase 2a Alzheimer’s disease study. The study will have 450 patients and will be held at 52 sites across North America, Europe and Australia.
ANAVEX®2-73-RS-001 is being evaluated in a Phase III clinical trial to treat pediatric patients with Rett syndrome and has shown statistically significant improvement in RSBQ (Rett Syndrome Behavior Questionnaire) scores and CGI-I scores, when compared to a placebo. The drug was found to be well -tolerated, safe and registered good patient compliance.
ANAVEX®2-73 is undergoing a phase II clinical trial for the treatment of Parkinson’s Disease Dementia (PDD) and has demonstrated significant dose dependent improvements in the quality of episodic memory. The Company’s pipeline consists of ANAVEX 3-71 intended for the treatment of Frontotemporal Dementia and neurodegenerative diseases; ANAVEX 1-41 for the treatment of Depression, Stroke and Neurodegenerative Diseases and ANAVEX-1066 for the treatment of Visceral Pain and Acute and Neuropathic pain.
The Company retains worldwide rights to all of its product candidates and has a strong intellectual property rights portfolio. Anavex has a cash runway of 36 months and access to non-dilutive cash resources such as the Michael J Fox Foundation, Rettsyndrome.org, Australian government.
Weakness
Anavex had a class action lawsuit filed against the company in February 2016, claiming it had artificially inflated stock prices, in violation of federal security laws. The lawsuit, which was filed in the U.S. District Court for the Southern District of New York, also contained allegations of misleading investors through false and misleading statements and the use of paid promotion and unusual market activity. Consequently, On June 5,2013, the British Columbia Securities Commission ordered the suspension of all trading in the securities of the Company.
However, the Company issued a clarification that it did not authorize, permit or participate in the promotion of its shares. Anavex also informed investors about some of the claims that may be exaggerated or inaccurate regarding its business prospects.
Based on the statement, the British Columbia Securities Commission passed a revocation order on June 20,2013.
In January 2017, the Company announced the dismissal with prejudice in its entirety of the Cortina v. Anavex Life Sciences Corp. et. al. lawsuit, with the judge refusing to allow the plaintiff to amend their complaint, proving the company’s conviction that the lawsuit was without merit.
Opportunities
Parkinson’s is the second most common age-related neurodegenerative disorder, characterized by muscle rigidity, bradykinesia, cognitive impairment etc. An estimated one million people in the USA are currently battling this disease, with over 60,000 new diagnoses each year. The cost of healthcare is a staggering $25 billion per year which includes treatment cost and lost income.
A report by market research engine projects a CAGR of 6% for the Parkinson’s disease treatment market, and expects it to reach US$ 6.0 Billion by 2024. A rise in geriatric population with growing incidences of Parkinson’s coupled with the emergence of novel combination therapies such as dopaminergic stimulation medicines, gene therapy and neural transplantation will help the market grow, while the prohibitive treatment costs and the introduction of generics may act as limiting factors.
Researchers have identified Alpha-synuclein (also α-synuclein) protein as the cause of this disorder though its role in brain regulation is unknown. Alpha-synuclein has been the focus of extensive research efforts to understand its role in Parkinson’s and as a prospective base for novel neuroprotective therapies.
Companies are increasingly exploring newer mechanisms of combating this illness and with emergence of novel technologies the focus has shifted to innovative treatment methods such as Gene therapy, peptide-based therapeutics, dopamine agonists and protein inhibitors. We take a look at a few such companies that are leading the innovation bandwagon.
Alzheimer’s is the sixth leading cause of death in the U.S, a disease that causes irreversible degeneration of the brain cells and affects a person’s cognitive and behavioral abilities such as impaired judgment, deteriorating memory and personality changes with the gradual loss of motor abilities and speech.
According to some compelling statistics made available by Alzheimer’s Association over 5.8 million Americans are affected by this disorder and the numbers will most likely reach 14 million by 2050, with a new diagnosis being made every 65 seconds. The healthcare cost of Alzheimer’s and other Dementia related ailments will be over $290 billion in 2019, these numbers are likely to escalate further to over $1.1 trillion by 2050.
Image Source: NIH
A report by transparency market research predicts that the global market for Alzheimer’s will reach US$6.4 billion by 2025, growing at a CAGR of 7.5%, from US$3.6 billion in 2017. The rise in ageing population, increased awareness about the benefits of combined therapy, a favorable regulatory and government support environment are some of the factors that will contribute to the growth of the market. The economic burden of the disease on not only the patients but also caregivers will act as an impediment for the market. The prohibitive cost of expensive treatment especially in developing nations is another obstacle for growth.
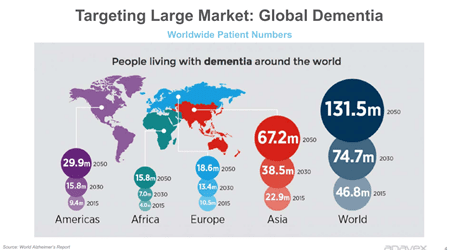
Image Source: Company
In June 2021, Biogen’s ADUHELM™ (aducanumab-avwa), scored an approval from the U.S. Food and Drug Administration (FDA), to become the first drug approved after 2003 for the treatment of Alzheimer’s disease, which specifically addresses the underlying cause of the disease, by removing amyloid beta from the brains of those afflicted with the condition.
The approval has met with criticism from several quarters which raises questions about its efficacy.
In March 2019, the Company had announced that it was halting two Phase 3 clinical trials of aducanumab, based on the results from an analysis conducted by an independent monitoring committee. The candidate was found to be unlikely to benefit patients when compared to a placebo. Aducanumab was believed to have the potential to remove beta-amyloid, a protein which is responsible for causing the damage. However, In October 2019, the Company decided to pursue a regulatory approval of the drug after discussion with the FDA and analysis of a larger data set, which showed reduced clinical decline in patients with early Alzheimer’s disease.
ANAVEX®2-73 is undergoing a phase II clinical trial for the treatment of Parkinson’s Disease Dementia (PDD) and has demonstrated significant dose dependent improvements in the quality of episodic memory
ANAVEX®2-73 has also demonstrated improved Mini Mental State Examination (MMSE) and Alzheimer’s Disease Cooperative Study Group – Activities of Daily Living Inventory (ADCS-ADL) scores through 148 weeks in a Phase 2a clinical study.
Threats
Alzheimer’s is one illness, where innumerable attempts have been made to find a cure with no success. According to a report released by Pharmaceutical Research and Manufacturers of America (PhRMA) from 1998 to 2017 there have been about 146 failed shots at developing drugs for Alzheimer’s disease. However recent advancements in technology and the discovery of new molecules and combination therapies are offering renewed hope to those suffering from this illness.
Clinical Trials are fraught with risk and uncertainty. Anavex is currently focused on ANAVEX®2-73 for the treatment of Alzheimer’s disease, Parkinson’s disease and Rett syndrome and ANAVEX®3-71 for frontotemporal dementia. However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to meet endpoints in any of its ongoing trials. The success of its clinical trials will help the Company advance its pipeline but it should also be prepared to face any setbacks, in case its ongoing trials fail to meet their endpoints.
Conclusion
We believe that Anavex Life Sciences has a potential blockbuster in the making, which can completely transform the Alzheimer Disease treatment landscape. The Company’s differentiated approach, promising clinical data and a pipeline of indications with large unmet medical need make it a winner!
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
http://phrma-docs.phrma.org/files/dmfile/AlzheimersSetbacksSteppingStones_FINAL_digital.pdf
https://www.transparencymarketresearch.com/pressrelease/alzheimers-drugs-market.htm
https://www.alz.org/alzheimers-dementia/facts-figures
https://robbinsllp.com/anavex-life-sciences-corp/
http://www.anavex.com/wp-content/uploads/2021/04/Anavex-Presentation-April-2021.pdf





No Comments