
30 Aug Beyond Air: Strong Upcoming Catalysts Make this a must watch!
Beyond Air, Inc. (NASDAQ: XAIR) formerly known as AIT Therapeutics, Inc, is a clinical-stage biopharmaceutical and medical device company that uses a proprietary technology to produce nitric oxide from ambient air through its LungFit System. The device enables the administration of ultra-high nitric oxide concentrations to direct tumors for treatment of respiratory conditions, severe lung infections and solid tumors.
The Company has submitted a premarket approval (PMA) application to the U.S. Food and Drug Administration (FDA) for use of the Company’s LungFit™ PH for the treatment of persistent pulmonary hypertension of the newborn (PPHN). The device is subject to a 180-day review period as per FDA guidelines. The technology has the potential to replace cumbersome nitric oxide cylinders and is looking at a potential launch in H2,2021.
Beyond Air, Inc. (NASDAQ: XAIR)
Market Cap: $272.77M; Current Share Price: 11.39 USD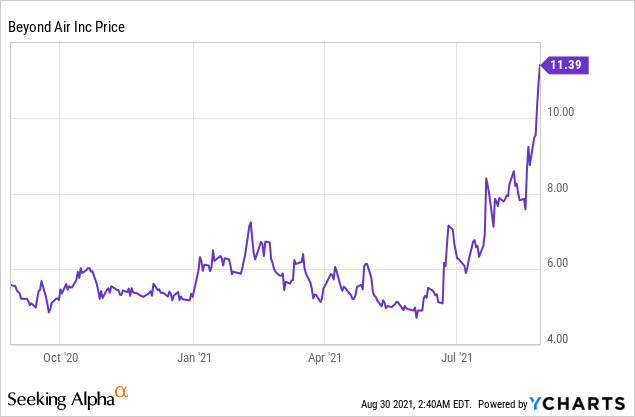
Data by YCharts
Technology
LungFit is a cylinder-free, phasic flow nitric oxide generator and delivery system that is ventilator compatible and can generate Nitric Oxide from ambient air at concentrations ranging from 1 part per million (ppm) to 80 ppm. The device provides a much-needed alternative to conventional large, high-pressure NO cylinders, by reducing inventory and storage requirements and simplifying and improving the delivery of NO based treatments. The Company is also seeking to expand to the home treatment market in the future.
The cutting-edge technology of the Company can generate up to 400 parts per million of nitric oxide, which can deliver NO to the patients’ lungs with the capacity to titrate on demand. The blend is administered through a ventilator or a face mask depending on the concentration. The device is user friendly and easy to understand and administer by medical staff.

Image Source: Company
The technology offers numerous benefits to hospitals as it eliminates the need for cumbersome storage and inventory management requirements, does not require additional NO2 buildup related safety measures or purging procedures and improves operational economies. Most importantly, its adoption does not require major capital investments for even hospitals that have not used NO previously.
Mallinckrodt’s INOmax has been the undisputed market leader since 1999 and generates more than $500 million in sales in the US alone. However, the monopoly was broken in 2019, with the entry of other competitors such as Vero’s GENOSYL and Praxair’s NOxBOX. However, the INOmax and NOxBOX systems are heavy, cumbersome to store and transport and potentially hazardous in case NO leaks out accidently. The GENOSYL uses cassettes of N204 liquid to convert liquid to NO, however these cassettes need to be stored at a specific temperature between 20-25 degrees and are toxic.
Pipeline
The Company’s research and development pipeline consists of an ongoing pilot study of LungFit PRO for the treatment of Acute Viral Pneumonia (including COVID), the interim data from which is expected to be released in the spring of 2021. Beyond Air is also evaluating LungFit GO for the treatment of Nontuberculous mycobacteria (NTM) lung infection, which is intended to be self-administered at home and is currently a subject of an ongoing pilot study. The interim data from the study is expected to be announced in mid-2021.

Image Source: Company
As part of the Company’s preclinical studies, Beyond Air is evaluating the efficacy of its technology in treating severe exacerbations due to lung infections in COPD patients and intends to initiate First in human trials in multiple solid tumors by the end of 2021.
The Company is also pursuing the treatment of Bronchiolitis, which is currently on hold, and intends to initiate a pivotal study in Q4,2022, based on the pandemic.

Image Source: Company
Industry
A baby generally receives oxygen from the mother and the placenta during pregnancy. The blood vessels in the lung are not fully operational and open up completely only after childbirth. However, in some newborns, the blood vessels do not open up and this leads to a limited supply of oxygen to the brain and other main organs. The disease affects 1.9 per 1000 live births with mortality rate ranging between 4–33% as per the Company.
Persistent pulmonary hypertension in the neonate (PPHN) forces the heat to use fetal circulation pathways that may allow low-oxygen blood to mix and contaminate the blood pumped to the rest of the body. The disorder manifests in the form of breathing difficulties, blue colored skin, low blood pressure and low blood oxygen levels. Although the exact cause of PPHN is unknown, it is believed to be caused by meconium aspiration, respiratory distress syndrome, lack of oxygen during birth and diaphragmatic hernia.
The current treatment options include supply of oxygen through special ventilators, use of intravenous medicine to support blood pressure, Opening the pulmonary vasculature through use of Nitric Oxide and extracorporeal membrane oxygenation (ECMO).
According to a report by FutureWise Market Research and Reports, the global market for Inhaled Nitric Oxide is projected to reach over USD 1.5 billion by 2027, growing at a CAGR of 7.8% from 2020.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.futurewiseresearch.com/healthcare-market-research/Inhaled-Nitric-Oxide/5595





No Comments