
21 Jun ChemoCentryx: A Lot Rides on the Approval of Avacopan
ChemoCentryx, Inc. (NASDAQ: CCXI) is a clinical-stage biopharmaceutical company that focuses on creating innovative therapeutics for treatment of autoimmune, inflammatory and oncology disorders with primary focus on orphan and rare diseases. The Company’s approach is centered on developing discrete chemokine or chemoattractant receptors that block the negative inflammatory or suppressive response while leaving the rest of the immune system intact.
Most recently the Company announced the outcomes from the Phase III ADVOCATE trial and Phase II ACCOLADE and LUMINA-1 trials at the ERA-EDTA (European Renal Association – European Dialysis and Transplant Association), held in Berlin, Germany. The Company’s presentation on ‘The Effect of Avacopan, a Complement C5a Receptor Inhibitor, on Kidney Function in Patients with ANCA-Associated Vasculitis with Renal Disease’ was selected as one of the 10 best extracts of the congress.
The data from the Phase III ADVOCATE trial shows that patients suffering from ANCA vasculitis with renal disease treated with Avacopan demonstrated greater recovery in estimated Glomerular Filtration Rate (eGFR) and a more rapid increase in the urine albumin-to-creatinine ratio than prednisone, basically showing its potential in lowering long-term end-stage renal failure and death.
In September 2020, ChemoCentryx submitted a New Drug Application (NDA) for Avacopan, a first-in-class small molecule, intended for the treatment of ANCA-associated vasculitis. The drug works by blocking the receptor responsible for the pro-inflammatory complement system fragment namely C5a, which is the primary cause of ANCA-associated vasculitis. The FDA has granted an orphan drug designation for Avacopan for the treatment of ANCA-associated vasculitis and C3G. In addition, the European Commission has granted the candidate an orphan medicinal product designation for two forms of ANCA-associated vasculitis: microscopic polyangiitis and granulomatosis with polyangiitis (formerly known as Wegener’s granulomatosis), as well as for C3G.
The FDA has set a July 7, 2021 Prescription Drug User Fee Act (PDUFA) goal date for the approval of the candidate.
ChemoCentryx, Inc. (NASDAQ: CCXI)
Market Cap: $ 942.32M; Current Share Price: 13.51 USD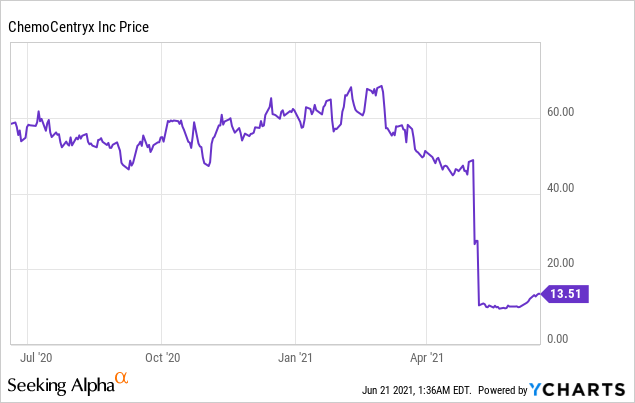
Data by YCharts
Industry
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of autoimmune disorders characterized by inflammation of small vessels and necrosis. The main reason behind this disease is believed to be overly active Antineutrophil cytoplasmic antibody (ANCA)-which binds to neutrophils and causes damage to the small blood vessels. According to the NCBI the disease can manifest in three forms namely granulomatosis with polyangiitis (GPA; formerly known as Wegener’s granulomatosis), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA; previously known as Churg-Strauss syndrome). The disease is more commonly found in the elderly and mostly affects men and if left untreated can prove fatal. It affects one in 50,000 people as per an estimate.
The disease can affect several key organs of the body such as kidney, lungs, stomach and intestines to name a few. Though there is no clear understanding of the reasons behind this autoimmune disorder, scientists believe that genetics and environmental factors such as certain drugs like cocaine, pesticides, exposure and inhalation of silica alcohol or glues and pollution play a key role in causing this disease.
Most common symptoms include vascular damage in the kidneys, high blood pressure, nerve problems such as tingling, numbness, burning sensation, cognitive impairment, respiratory tract issues and gastrointestinal problems among others. Physicians may use various tests to determine if a patient is suffering from vasculitis and the subtype. In addition, there are blood tests, tissue analysis and biopsies which can help diagnose the disease, besides scans, computed tomography, bronchoscopy and nasal endoscopy.
There is no cure for the disease currently, however treatment is aimed at alleviating symptoms and preventing remission and includes administration of glucocorticoids, cyclophosphamide and other autoimmune drugs.
Company
The Company is targeting orphan and rare diseases in the field of renal, immuno-oncology, inflammatory and autoimmune diseases. ChemoCentryx’s approach is based on focusing on a specific chemokine or chemoattractant receptor that can inhibit negative inflammatory response, with no impact on the immune system.

Image Source: Company
It has an extensive pipeline covering areas such as C3 Glomerulopathy, Hidradenitis Suppurativa (Avacopan / CCX168)/ C5aR), CCX140/ for Diabetic Nephropathy which has successfully completed a phase II trial, CCX507/ CCR9, CCX587/ CCR6 for autoimmune diseases such as Ulcerative Colitis and Th17 driven diseases.
The Company has established a Kidney Health Alliance with Vifor Pharma (VIFN.SW) and has entered into a joint development agreement with the company to develop CCX140 for renal diseases. As part of the agreement, it will retain the marketing rights for the U.S. and China while Vifor will exercise rights over the rest of the markets worldwide. ChemoCentryx has received $155 million in upfront cash commitments, in addition to $1.2 billion in potential development, regulatory and sales milestone payments plus royalties in the mid-twenties on net sales in the Vifor Pharma territories.

Image Source: Company
Key Takeaway
The Company is facing a lawsuit over alleged misrepresentation of facts / failure to disclose to investors information such as issues about the interpretability of data from the Phase III trial of avacopan owing to study design and serious safety concerns about Avacopan, which raised questions about the viability of its New Drug Application for Avacopan for the treatment of ANCA-associated vasculitis. These issues were raised in a briefing document published by the FDA in May 2021.
However, the FDA arthritis committee voted 10-8 in favor of the 30 mg dose of Avacopan, stating that the safety and risk-benefit profiles for the drug were sufficient to support approval. In addition, the committee was divided on its decision whether the efficacy data supported the approval of Avacopan for the treatment of adult patients with ANCA-associated vasculitis. The decision was based on the ADVOCATE phase 3 trial results which evaluated Avacopan among adult patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) and met its primary endpoints. The FDA however, has raised concerns about the interpretability of the data and that the noninferiority data was insufficient to conclude that Avacopan could act as replacement for glucocorticoids.
The FDA is likely to announce its final decision on July 7,2021 and is not necessarily required to follow the recommendations of the advisory committees. Avacopan is also being reviewed by the European Medicines Agency and is expected to announce its decision in H2,2021.
The approval of Biogen’s Aduhelm, with a far more critical committee, may point to much better chances of an approval for Avacopan. According to Raymond James, the drug has a 50/50 probability of approval, most likely after a 3-month extension as against the July 7 PDUFA. The Company is taking a proactive approach by clarifying data and providing additional information about the drug to the FDA.
ChemoCentry is planning to initiate clinical development of orally administered PD-1/PD-L1 inhibitor CCX559, a candidate targeting oncology indications in H1,2021 and is targeting potential fast track designation, which would expedite the approval and regulatory process.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6297586/
https://ancavasculitisnews.com/what-is-anca-vasculitis/
https://rarediseases.info.nih.gov/diseases/13011/anca-associated-vasculitis
https://ir.chemocentryx.com/static-files/b5deabc3-8897-4b9f-b8b3-ba6aff8ef62b





No Comments