
26 Jan Emerging Paradigms in treatment of Melanoma!
Melanoma, a form of skin cancer, accounts for about 1% of skin cancers but is responsible for over 7,650 deaths every year as per an estimate by the American Cancer Society, these numbers are steadily rising over the years with approximately 99,780 new melanomas diagnosis being made each year.
The 5-year survival rate for this illness has shown a significant improvement with faster and timely diagnosis, aggressive skin cancer screening programs for populations at greater risk of contracting the disease and rapid advancements in surgical and therapeutic options.
According to a report by Global data the global treatment market for melanoma is expected to reach $7.42bn in 2029 from $5.59bn in 2019 growing at a CAGR of 2.9%. The major drivers for growth in the market would be the expanding treatment options to include targeted combination therapies, deteriorating environmental condition leading to higher exposure to UV rays, favorable government and regulatory environment, patent expiration of certain biosimilars and launch of generics that are expected to pave the way for new players to enter the market.
However, the prohibitive cost of treatment may prove challenging and affect the growth prospects to an extent. A number of clinical trials are underway in the melanoma treatment industry with companies introducing unique first-in-class treatment options, some of which are enumerated below:
Nektar Therapeutics (NASDAQ: NKTR)
Market Cap: $2.01B; Current Share Price: 10.90 USD
Data by YCharts
Nektar’s areas of focus are cancer, auto-immune disease and chronic inflammatory diseases, where the company seeks to make an impact with its novel Polymer chemistry platform. The company’s lead drug candidate for treatment of melanoma is an immunostimulatory IL-2 cytokine prodrug + anti-PD-1 named Bempegaldesleukin (+ OPDIVO®), which is currently undergoing Phase 3 registrational study (PIVOT-IO-001) in partnership with Bristol Myers Squibb.
Bempegaldesleukin (BEMPEG/NKTR-214) has the potential to stimulate and expand specific cancer-killing T-cells and natural killer (NK) cells, without affecting intratumoral regulatory T cells. The candidate can deliver a controlled, sustained, and preferential IL-2 pathway signal and is part of five registrational programs and one mid-stage program for BEMPEG in combination with nivolumab (OPDIVO®) that the company is developing in collaboration with BMS.
The Company is currently engaged in a phase 3, randomized, open-label study of bempegaldesleukin (BEMPEG; NKTR-214) plus nivolumab (NIVO) versus NIVO monotherapy for the adjuvant treatment of patients with completely resected melanoma at high risk of recurrence. The trial will enroll 950 patients over the age of 12 with Respected Stage IIIa–IV melanoma with no evidence of disease. The primary endpoint of the study is Recurrence-free survival, while secondary endpoints include overall survival, distant metastasis-free survival, safety and tolerability and quality of life.
The candidate has demonstrated durable responses and encouraging clinical activity in PIVOT-02, a multicentre phase 1/2 study in multiple solid tumor settings. The combination showed clinical activity with ORR 53% and CR 34%, in efficacy-evaluable patients, demonstrated durable and deep responses over time and was well-tolerated. The combination of BEMPEG + NIVO was granted a breakthrough therapy designation for treatment of patients with previously untreated, unresectable or metastatic melanoma by the FDA in July 2019.

Image Source: Company
BEMPEG is also undergoing evaluation in numerous indications including genitourinary cancer, (metastatic renal cell carcinoma), muscle-invasive bladder cancer, adjuvant melanoma metastatic head and neck cancer whose tumors express PD-L1, cancer vaccines, treatment for mild COVID-19 among others. In addition, Nektar is also developing NKTR-358 for autoimmune diseases (Systemic Lupus Erythematosus), NKTR-358 for ulcerative colitis, NKTR-358 for psoriasis and NKTR-358 for Atopic Dermatitis in association with Lilly.
The Company has multiple clinical collaborations, co-development and licensing agreements with top-notch Biotechnology and pharmaceutical companies such as Bristol-Myers Squibb, SFJ pharmaceuticals, Merck, Exelixis, Vaccibody, Lilly and Janssen to name a few, which are leveraging the company’s proprietary platform to develop next-generation therapeutics.
MacroGenics Inc (NASDAQ: MGNX)
Market Cap: $782.27M; Current Share Price: 12.77 USD
Data by YCharts
Macrogenics is developing monoclonal antibody-based therapeutics by leveraging a proprietary next-generation antibody-based technology platform namely DART and Trident and its expertise in proteins. These platforms enable the creation of single bispecific molecules that are capable of binding with one or two or more targets separately as a combination. The platform can overcome the limitations of conventional engineering challenges such as instability, short half-lives and manufacturing inefficiencies.
The Company’s lead candidate for Melanoma is MGC018, an investigational antibody-drug conjugate (ADC) that was licensed from Byondis, B.V. The candidate is designed to target solid tumors metastatic castrate-resistant prostate cancer (mCRPC), non-small cell lung cancer (NSCLC), and triple-negative breast cancer (TNBC).

Image Source: Company
The Company already has a product in the market named Margenza, a HER2/neu receptor antagonist indicated, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease.
Macrogenics is developing a diverse pipeline of candidates that include Enoblituzumab
(B7-H3) intended for the treatment of SCCHN (Head & Neck Cancer) (+retifanlimab/tebotelimab), Flotetuzumab (CD123 × CD3) for treatment of Refractory AML and MGD024 (CD123 × CD3) targeting CD123+ Heme Malignancies among others.
The Company has established multiple strategic partnerships and collaborations with companies like Incyte Corporation ( development and commercialization of MGA012, an anti-PD-1 antibody), I-Mab Biopharma ( development and commercialization rights in mainland China, Hong Kong, Macau and Taiwan for enoblituzumab), GC Pharma (Korea) ( development and exclusive commercialization of margetuximab in South Korea), Zai Lab Limited (margetuximab, MGD013 and an undisclosed multi-specific TRIDENT™ molecule) and Janssen for a preclinical bispecific molecule targeting of two undisclosed targets outside oncology) to name a few.
CytomX Therapeutics Inc (NASDAQ: CTMX)
Market Cap: $285.14M; Current Share Price: 4.37 USD
Data by YCharts
The Company’s lead candidate for Metastatic Melanoma is BMS-986249, a Probody version of the anti-CTLA-4 antibody Yervoy® (ipilimumab), in combination with Opdivo® (nivolumab) in patients with metastatic melanoma that is currently undergoing first-in-human Phase 1/2a trial. The candidate is being developed in collaboration with Bristol Myers Squibb, which has initiated a Phase 2 randomized 5-arm cohort expansion study. In addition, the Company is developing BMS-986288, a second anti-CTLA-4 Probody, based on a modified version of Yervoy® (ipilimumab), which is currently being evaluated in patients with selected advanced solid cancers as a standalone therapy or in combination with Opdivo® (nivolumab).
CytoMx is using its expertise in Probody® therapeutics to create treatments that have a wider therapeutic window, generate new combinations that target “undruggable” conditions and limit toxicity. Probody therapeutics only bind to tumors and leave the healthy tissue unaffected. The Probody® platform can be applied to Immunotherapies, antibody drug conjugates, T cell engaging bispecifics, and cytokine therapy.
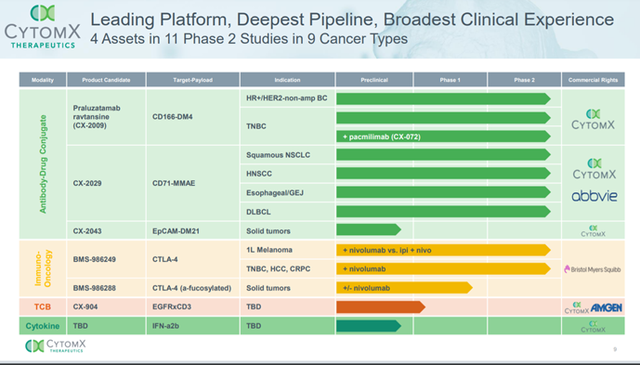
Image Source: Company
Besides BMS-986249, the Company’s pipeline consists of CX-2009, a CD166 Probody drug conjugate (PDC) intended for the treatment of ER/PR Positive, HER2 Negative Breast Cancer, CX-072 + CX-2009, a PD-L1 Probody immunotherapy (IO) + CD166 PDC for the treatment of TNBC that the Company is developing on its own. Cytomx is also developing CX-2029, a PDC directed against CD71, along with Abbvie. The candidate is currently undergoing Phase 1/2 clinical trial as monotherapy.
The Company has entered into collaboration with Amgen for developing CX-904, a T-cell engaging bispecific Probody candidate against Epidermal Growth Factor Receptor (EGFR) and CD3 and has another preclinical CD3-TCB that is being developed in association with astellas and Amgen.
CytomX has built a strong intellectual property rights portfolio with over 450 issued and pending patents worldwide and has entered into 4 global partnerships and 3 partnered programs. The Company has 4 candidates in Phase 2 across 9 cancer types. Upcoming catalysts in 2022 include initial phase 2 data in breast cancer, completion of expansion phase for CX-2029 and initiation of Phase 1 study of CX-904.
Ideaya Biosciences Inc (NASDAQ: IDYA)
Market Cap: $621.73M; Current Share Price: 16.15 USD
Data by YCharts
Ideaya, a precision-medicine oncology company is evaluating darovasertib and crizotinib synthetic lethal combination in metastatic uveal melanoma (MUM) patients in a phase 1 / 2 trial. In December 2021, the Company announced a clinical update, which demonstrated a new clinical efficacy benchmark and clinical proof-of-concept for the PKC and cMET synthetic lethal combination, serving as a validation of its synthetic lethality platform.
Darovasertib (IDE196) is a potential first-in-class PKC inhibitor that is being developed by the Company under a clinical trial collaboration and drug supply agreement with Pfizer. The target enrollment for the trial is approximately 40 patients in an ongoing Phase 1/2 clinical combination arm. The preliminary data suggests a 100% disease control rate, 31% overall response rate and >30% tumor reduction in 46% of patients (6 of 13) with > 2 post-baseline scans. The Company will be using the darovasertib and crizotinib combination expansion dose to support potential registrational studies and is targeting regulatory feedback in the 2022.
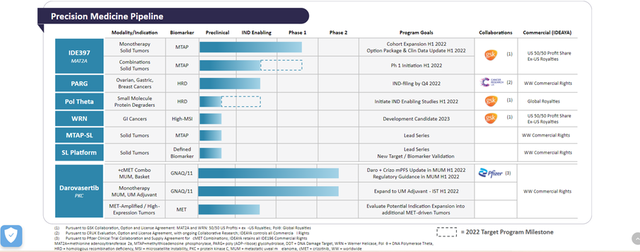
Image Source: Company
The Company intends to explore the application of PKC and cMET synthetic lethal combination in other potential tumor settings like GNAQ/11 skin melanoma and MET-amplified and MET high expression tumors.
Furthermore, the Company’s pipeline consists of MAT2A, intended for the treatment of solid tumors, which is being developed in collaboration with GlaxoSmithkline. The Company is also working on IND enabling studies for Pol Theta, a small molecule protein degrader and WRN for GI cancer in collaboration with gsk. In addition, the pipeline also has PARG for treatment of ovarian, gastric and breast cancers which is being developed in association with Cancer Research UK.
Market Cap: $4.07B; Current Share Price: 25.19 USD
Data by YCharts
A biopharmaceutical company that is focused on addressing the needs of patients suffering from debilitating diseases, Alkermes is currently undertaking research on disorders of the Central Nervous System (CNS) such as Multiple Sclerosis, Schizophrenia, depression, addiction and cancer.
In April 2021, the Company had announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track designation to nemvaleukin alfa (nemvaleukin), an interleukin-2 (IL-2) variant immunotherapy, intended for the treatment of mucosal melanoma. The candidate was also granted an orphan drug designation earlier. Nemvaleukin is currently undergoing a global phase 2 trial namely ARTISTRY-6 evaluating the candidates in patients with melanoma who have been previously treated with anti-PD-(L)1 therapy.
The other candidates in the Company’s pipeline include Nemvaleukin alfa (in combination with pembrolizumab for treatment of Platinum-Resistant Ovarian Cancer), ALKS 1140 (Neurology) and ALKS 2680) Narcolepsy.

Image Source: Company
The company’s forte is its long-acting technological platform namely Medisorb®, LinkeRx® and NanoCrystal® that enable the gradual release of small and macromolecules at a controlled rate over a monitored period of time which reduces the need for frequent medication and improves patient adherence. Alkermes Oral Controlled Release technology allows it to customize the dosage of a drug to achieve immediate release; oral delayed release, extended release, pulsatile release, chono-timed release or a combination of the above, thereby overcoming the limitations of existing treatment options.
In addition, the company is also developing Nanoparticle Technology platforms called the NanoCrystal® technology and the NanOsmotic® technology that improve the bioavailability of drugs with poor water-solubility, reduce dosage volume and maximize dosage tolerance. The company is also working on drug candidates that target opioid receptors that have the ability to affect addiction, behavior and mood modification, immunology, and pain. The Company’s proprietary Medifusion™ technology deals with protein and peptides and modifies their circulating half-life to deliver long-acting injectable medications.
The company has dedicated GMP manufacturing facilities in Ohio and Ireland that are FDA / EMA approved.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.nektar.com/pipeline/rd-pipeline/nktr-214
https://www.nektar.com/application/files/4316/2852/5677/NEKTAR_PIVOT-12_Flashcard_-_July_2021.pdf
https://macrogenics.com/mgc018-b7-h3/
http://ir.macrogenics.com/static-files/4188cbdd-3956-4297-aaaa-fc769b4074e2
https://ir.cytomx.com/static-files/8342f2b5-9fa5-462b-97bd-505f1a7e4c30
https://www.alkermes.com/research-and-development#pipeline
https://investor.alkermes.com/static-files/b634427a-7fbf-4e1f-81e6-ef2987bad9ed





No Comments