
13 May Heron Therapeutics: Impending Catalysts Makes It A Must Watch!
Heron Therapeutics, Inc. (NASDAQ: HRTX) has a major upcoming catalyst in the form of the upcoming Prescription Drug User Fee Act (PDUFA) date for FDA approval on May 12, 2021 for HDX-011 (Zynrelef). HTX-011 is an extended-release, dual-acting local anesthetic that uses a combination of bupivacaine with meloxicam, and is intended to minimize the need for opioid analgesics and reduce postoperative pain for 72 hours. The candidate has been granted a Fast-Track Designation, Breakthrough Therapy Designation and Priority Review by the FDA.
Heron Therapeutics already has two FDA-approved products in its portfolio SUSTOL (granisetron), an extended-release injection of serotonin-3 (5-HT3) receptor antagonist, indicated to be used with other antiemetics in adults undergoing moderately emetogenic chemotherapy (MEC) or anthracycline and cyclophosphamide (AC) and CINVANTI (aprepitant), a P/neurokinin-1 (NK1) receptor antagonist, in an injectable emulsion form, indicated to be used with other antiemetic agents for prevention of acute and delayed nausea and vomiting in cases of highly emetogenic cancer chemotherapy (HEC) or moderately emetogenic cancer chemotherapy (MEC).
Heron Therapeutics is gearing up for the potential launch of HTX-011 and plans to leverage its existing established platform that has experience with products like CINVANTI and SUSTOL to successfully introduce the product to the market. The Company has already proved its mettle in execution, with the launch success of CINVANTI in January 2018, and aims to replicate the same with HTX-011.
Heron Therapeutics, Inc. (NASDAQ: HRTX)
Market Cap: $ 1.77B; Current Share Price: 17.41 USD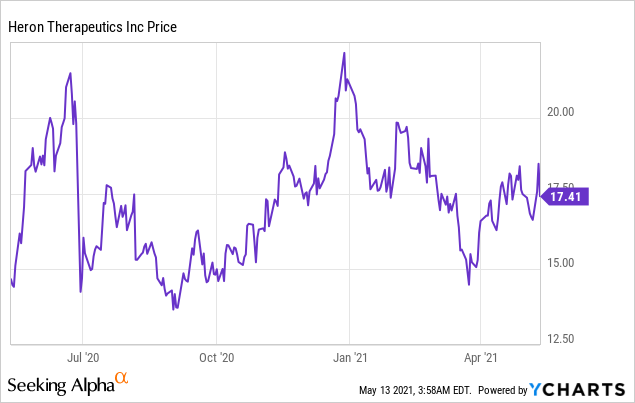
Data by YCharts
Industry
Most surgeries have some component of postoperative pain, which usually occurs at the incision site and can last from 2 to 15 days depending on the type of surgery and the depth of the incision. According to information from the National Hospital Discharge Survey, there were 42.5 million procedures that required patient hospitalization during 2020 in the U.S alone and there were many more surgeries performed on outpatients as well. The data also shows that more than 88 percent of the patients will have some form of postoperative pain, with 34–62% likely to experience moderate to severe postoperative pain.
Postoperative pain management is extremely crucial as mismanagement or undertreatment may result in sleep disturbances, tiredness and fatigue, delayed healing, extended hospitalizations and development of psychological complications such as depressive disorders or even chronic pain conditions. Analgesics are considered to be the best way to treat and manage postoperative pain and include drugs such as opioids, non-specific nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 (COX-2)-specific inhibitors, and local anaesthetics.
The global analgesic market is poised to reach $ 26.4 billion by 2022, growing at a CAGR of 7.1% during 2015 – 2022, according to a report published by Allied Market Research.
The Analgesic market is broadly divided into Opioid and Non-Opioid therapeutics. Opioid based medicine has given rise to an addiction endemic with prescription drug overdose becoming one of the leading causes of preventable deaths in the U.S in turn prompting the government to declare a public health emergency in 2017.
This has resulted in growth in the non-opioid based drugs segment, with increasing emphasis being laid on research and development in finding non-opioid alternatives to managing chronic pain. According to a study published by BBC research the global market for non-opioid pain treatment which was $ 9.9 billion in 2017 , is estimated to grow at a CAGR of 18% and reach $ 22.6 billion by 2022.
The largest product category under non-opioid pain treatment is that of Medical cannabis constituting 73% of the market, followed by Menthol – Containing treatments accounting for 9% of market share. Omega-3 fatty acid-based treatments constitute 9% of the market share, followed by Botulinum toxins-based drugs that contribute 5% and Capsaicin derived treatment make up 3% of the market share.
An increase in the geriatric population along with a rise in life-style related ailments and chronic diseases will drive the growth in the non-opioid market, along with a favourable government and regulatory environment, increased spending on research and development and fast track approvals for breakthroughs.
Company
The Company’s Biochronomer drug delivery technology has polymers, which when injected into the subcutaneous tissue, undergo controlled hydrolysis and enable controlled, sustained diffusion of the pharmacological agent and are gradually eliminated from the body. The technology lends itself to wide-scale adaptation in an array of therapeutic areas and can even accommodate multiple pharmacological agents in one Biochronomer polymer facilitating a multimodal therapy.

Image Source: Company
The Company had submitted a New Drug Application (NDA) for HTX-011 to the FDA in October 2018 and a marketing application in 2019. The Company received a Complete Response Letter (CRL) in June 2020 seeking additional non-clinical information. The candidate has already been granted a marketing authorization by the European Commission. HTX-011 for the management of postoperative pain has received a priority review status by Health Canada in 2019 and the Company is working on responding to questions raised by them.

Image Source: Company
Heron has two other candidates in the pipeline that are currently undergoing clinical development. HTX-019 (aprepitant) is an injectable emulsion intended for the management of post-operative nausea and vomiting. The Company intends to file an NDA for the drug in Q4,2021.
HTX-034 (bupivacaine, aprepitant and meloxicam) an extended-release solution is currently under clinical development for postoperative pain for 5 to 7 days. The candidate is being evaluated in a phase 2 clinical study for postoperative pain through local application into the surgical site.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.sciencedirect.com/science/article/pii/B9780721628721500157





No Comments