
11 May 4 Biotech Companies for your Investment Watchlist!
Biotech Companies continue to show resurgence even in these challenging times, with more and more companies persevering with bringing their clinical candidates to fruition. While Vaccine development and therapeutics to combat the COVID-19 pandemic are at the forefront of development activities in the biotech sector, there are some companies that are making strides in other related fields such as oncology, MPS and diagnostics. We bring to you some companies that are leveraging their leading-edge technology to make innovative therapeutics in areas that have a large unmet need.
iBio, Inc. (NYSE: IBIO)
Market Cap: $ 356.41M; Current Share Price: 1.65 USD
Data by YCharts
iBio is a leading biologic contract manufacturer which is currently developing IBIO-201, a SARS-CoV-2 vaccine candidate that uses its patented LicKM™ booster molecule, in combination with antigens derived from the spike protein. The Company has reported that the candidate has completed IND-enabling toxicology studies, which demonstrated no adverse effects at low or high doses. In addition, iBio is also working on IBIO-202, a subunit vaccine candidate that targets new viral variants by acting on the nucleocapsid protein of SARS-CoV-2.
The Company’s FastPharming® system is an amalgamation of vertical farming, automated hydroponics, and novel glycosylation technologies that enable it to produce high-quality monoclonal antibodies, vaccines, bioinks and other proteins. Furthermore, the iBio CDMO LLC, the Company’s subsidiary offers Contract Development and Manufacturing Services, which includes Glycaneering™ Development Services for advanced recombinant protein design.
iBio believes that a wide range of proteins can be expressed using its Nicotiana benthamiana plants, which are also scalable to produce high quantities in the company’s 130,000-square-foot FastPharming Facility in Bryan, Texas. The unique system provides several advantages such as greater speed-to-clinic, lower contamination risks, avoiding plastic usage, ease of scalability and better performance.
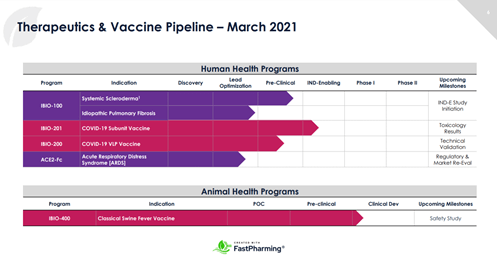
Image Source: Company
The Company is also developing IBIO-100 intended for the treatment of Idiopathic pulmonary fibrosis [IPF] and Systemic sclerosis. The candidate is a fusion of the endostatin derived E4 antifibrotic peptide to the hinge and heavy chain of human IgG1 and is based on the work of Dr. Carol Feghali-Bostwick. IBIO-100 has been granted an orphan drug designation by the US FDA for systemic scleroderma indication and is currently undergoing development to support an Investigational New Drug (IND) application.
IBIO-400, a differentiation of infected from vaccinated animals (DIVA) candidate, intended for the Classical swine fever (CSF) in feral and domesticated pigs, is being developed by the company in collaboration with the Institute of Infectious Animal Diseases (IIAD) and Kansas State University.
Furthermore, the Company has organized a consortium for COVID-19 vaccine development with partners such as Infectious Disease Research Institute (IDRI), Texas A&M University (TAMUS), MRI Global, IBM Watson Health and The Beck Group. The Company has a robust patent portfolio with over 98 issued patents and 20 patent applications pending worldwide.
iBio announced the settlement of a lawsuit with Fraunhofer USA, Inc. which began in March 2015. As part of the settlement iBio is eligible to not only receive an initial payment that will cover its legal fees and expenses, but also entails additional cash payments, which are due in March 2022 and March 2023. The parties agreed to iBio’s ownership of intellectual property pertaining to certain plant-based biopharmaceutical production, and Fraunhofer USA has been granted a fully paid-up license to use the recombinant protein manufacturing technologies, as part of the settlement.
Precipio, Inc. (NASDAQ: PRPO)
Market Cap: $ 80.51M; Current Share Price: 4.44 USD
Data by YCharts
Precipio delivers specialized diagnostic services such as primary diagnostic services for cancers, secondary opinion services through SmartPath, HemeScreen, a hematologic malignancy screening panel and ICE COLD-PCR (MX-ICP) for liquid biopsies. The Company offers the HemeScreen product range which includes a CLL panel, AML panel, MPN panel and a proposed Anemia Panel.
The Company recently launched its COVID-19 rapid antibody test (20 minute) that is capable of detecting IgG & IgM antibodies on Amazon.com. The test was the first US-based test to receive emergency use authorization (EUA) for point-of-care diagnosis and is being manufactured by Nirmidas Biotech. This enables qualified medical point-of-care (POC) providers like physicians and registered medical facilities to purchase the tests directly and receive them within 2 business days.
The 20-minute test can help determine if an individual has developed antibodies following vaccination or exposure to the Covid-19 virus. This would help detect if a person is infected and analyze the efficacy of vaccination for the individual. The deal with amazon will help provide access to quick and efficient testing for medical practitioners, governments and states and enable policy decisions with respect to preventive measures, guidelines and lockdowns. The Company intends to provide over-the-counter access to the kit in the long-run, which offers a potential upside for investors.
However, with a majority of the population now being vaccinated and the chances of developing herd immunity being real, the diagnostics test kits may not enjoy the demand it forecasts in the long-run. The Company recently announced that the revenue from its HemeScreen POL (Physician Office Laboratory) testing system is likely to exceed $1M by Q4-2021. In addition, the Company has a pipeline of over 30 new customers across the country and is upbeat about its product revenues reaching 50% of pathology revenue by Q4,2021.
Furthermore, Precipio is working on expanding its product offering to include more panels for detecting various cancers, which will improve its bottom line and increase its average revenue per customer. With the opening up of the economy, the Company is focused on building robust relationships for its oncology sales pipeline. In January 2021, the company announced the signing of contracts with two of the largest oncology practices in the US namely West Cancer Center of Memphis and New York Cancer and Blood Specialists in New York.
Senseonics Holdings, Inc. (NYSE: SENS)
Market Cap: $ 770.25M; Current Share Price: 1.80 USD
Data by YCharts
Senseonics Eversense is the only-FDA approved, long-term continuous glucose monitor which offers features such as an implantable sensor, a mean absolute relative difference (MARD) of 8.5 percent, detachable transmitter, MRI compatible sensor among others. The system was approved by the FDA in 2018 and has constantly been adding new features and functionalities. The Company is anticipating the approval of its New Eversense 180-day sensor and is preparing a submission for Investigational Device Exemption (IDE) for its 365-day sensor.
In August 2020, the Company also entered into a strategic partnership with Ascensia Diabetes Care, the global leader in blood glucose monitors. Ascensia has signed a commercialization and collaboration agreement with the company, which makes it the exclusive worldwide distributor for Senseonics’ Eversense® CGM systems. Senseonics has also entered into a concurrent financing agreement with PHC Holdings Corporation, the parent company of Ascensia for up to $80 million of debt and equity capital.
The deal helps to combine the relative strengths of both the companies, with Ascensia having more than 80 years of experience in diabetic care products and a presence in more than 125 Countries worldwide and global sales of approximately $1B. Senseonics will retain all product development, branding, regulatory approval and manufacturing responsibilities. The Companies are gearing up for the launch of New Eversense 180D (US and Europe), which is pending FDA approval, by preparing the groundwork for launch and patient initiation.
The Company has extensive commercial and medicare coverage with companies like Aetna, Bluecross Blueshield Horizon NJ health, Tricare and the U.S Department of Veteran Affairs among others, with over 200 million lives covered since its launch in 2018.
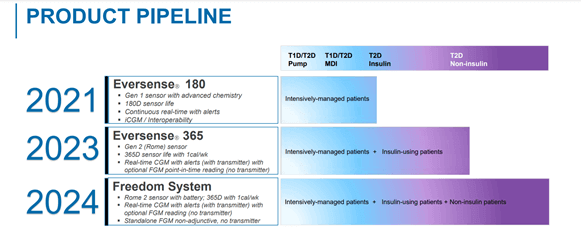
Image Source: Company
According to the International Diabetes Federation, the number of people living with diabetes will rise to 629 million by 2045, from 425 million adults (20-79 years) in 2017. In the U.S alone the people living with diabetes are set to increase from 58 million in 2017 to 67 million by 2045. Non-Invasive blood glucose monitoring offers a painless and more convenient alternative to monitoring glucose levels. According to a report by Coherent Market Insights, the global market for non-invasive blood glucose monitoring devices which were worth over US$ 5.1 million in 2017 will grow at a CAGR of 35.0% from 2018 – 2026. The ease and convenience of using the device, elimination of costly finger sticks and speedy transfer and sharing of data are some of the drivers of popularity of these devices, while the point of accuracy being lesser than conventional glucose monitoring devices may hamper its growth.
Abeona Therapeutics, Inc. (NASDAQ: ABEO)
Market Cap: $ 141.63M; Current Share Price: 1.35 USD
Data by YCharts
A clinical-stage Biopharmaceutical Company, Abeona Therapeutics Inc is developing an AAV-based gene therapy named ABO-102, which has received an orphan drug designation, rare pediatric disease, RMAT and Breakthrough therapy designation from the FDA. ABO-102 uses an AAV9 vector platform to deliver a functional copy of the defective SGSH gene to cells in the central nervous system. It is designed to be one-time gene therapy that will help the body generate its own enzyme to break down glycosaminoglycans. The Company is currently conducting a Phase I/ 2 trial for high-functioning patients, with plans to conduct another trial for patients with advanced forms of the disease as well.
The Company is also working on creating therapeutics for Epidermolysis Bullosa (EB) and Batten disease. EB-101, its lead candidate for the treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB) is currently enrolling patients in a crucial Phase III clinical trial named “VIITAL”. The candidate has the potential to be the first approved therapy for RDEB and has demonstrated the ability to provide durable treatment for large chronic wounds, for up to 5 years of follow-up. The candidate has been granted Regenerative Medicine Advanced Therapy, Breakthrough Therapy, and Rare Pediatric Disease designations and Orphan Drug designation in both the U.S. and EU as per the Company. The Company is expecting topline results from the VIITAL™ study in late-2021 and is simultaneously working on completing enrollments in the ABO-102 MPS IIIA and ABO-101 MPS IIIB studies.
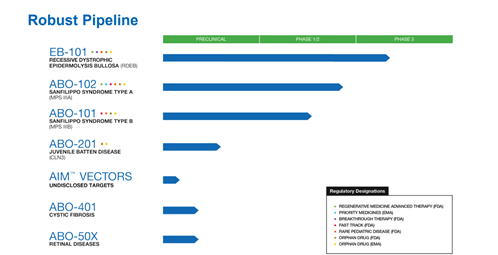
Image Source: Company
ABO-002 is designed to use an AAV9 vector platform, to deliver a functional copy of the PPT1 gene, the defective CLN1 gene, using a combination of intravenous and intrathecal administrations. This can promote proper metabolism in lysosomes by activating the PPT1 enzyme. Results from preclinical trials have demonstrated the safety and tolerability of the drug, along with improvement in survival rates, motor function and cognitive abilities in mice. The drug has been awarded an Orphan Drug Designation and a rare pediatric disease designation in the U.S, in addition to an Orphan Drug Designation in the E.U.
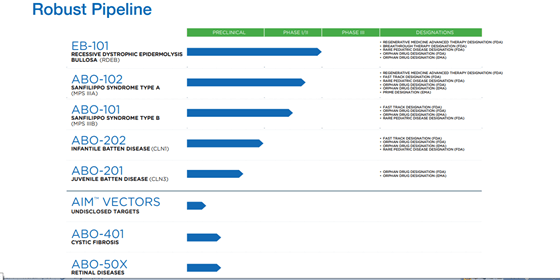
Image Source: Company
The Company has a state-of the-art cell and gene manufacturing and production facility to address its needs. Its pipeline also consists of candidates for the treatment of Cystic Fibrosis (ABO-401) and Retinal Diseases (ABO-50X). Furthermore, it is working on an undisclosed target named the AIM Vectors, touted to be a next generation AAV product. The AIM vector platform is next-generation AAV capsids that are non-replicating and can selectively target delivery of genetic material to the CNS, lungs, eye, muscle, liver and other tissues as per the Company. The Company’s AIM Capsid library has more than 100 capsids with tissue tropisms, having the potential to target numerous organs and routes of delivery.
The success of any cell and gene therapy is dependent on having a robust manufacturing capability, which allows stringent control on supply, costs and quality. The Company not only has a fully-operational, cGMP-compliant in-house manufacturing facility but has also tied up with The Elisa Linton Center for Rare Disease Therapies for clinical and commercial production of its adeno-associated virus (AAV)-based gene therapies.
The Company is building a diverse pipeline focused on using a range of delivery platforms and technologies such as autologous, gene-corrected cell therapy, one-time gene therapies using the AAV9 vector and a preclinical pipeline based on its AIM™ AAV platform. Some of the areas that Abeona is working on are cystic fibrosis, retinal disorders, RDEB, Infantile and Juvenile Batten Disease, which have a large unmet medical need and present an excellent opportunity for the Company.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.senseonics.com/investor-relations/news-releases/2020/08-10-2020-210456046





No Comments