
04 Feb Will Resmetirom Prove to be a Gamechanger in NASH?
Madrigal Pharmaceuticals, Inc. (NASDAQ: MDGL), a clinical-stage biopharmaceutical company, is developing novel therapeutics that target a specific thyroid hormone receptor pathway in the liver, an approach that has the potential to address the unmet needs in cardiovascular, metabolic and fatty liver diseases.
The Company’s research is focused on unraveling the underlying cause of Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) and potentially reduce the risk of cardiovascular morbidity caused by these disorders. Madrigal’s approach is based on thyroid hormone regulation of lipid metabolism by selective activation of thyroid hormone receptor beta (THR)-β.
Madrigal Pharmaceuticals, Inc. (NASDAQ: MDGL)
Market Cap: $1.13B; Current Share Price: 66.11 USD
Data by YCharts
Strength
The Company announced positive topline results from a Phase III trial evaluating its lead candidate Resmetirom (MGL-3196), in non-alcoholic fatty liver disease (NAFLD) and Non-alcoholic steatohepatitis (NASH). The results demonstrate that the candidate was safe, well-tolerated and showed statistically significant improvements on key parameters of Liver and Cardiovascular Health. Resmetirom was able to achieve the primary and secondary endpoints of safety and tolerability at 80 and 100 mg in patients treated for 52 weeks and showed clinically relevant reductions in liver fat as measured by magnetic resonance imaging proton density fat-fraction (MRI-PDFF), besides reducing atherogenic lipids, including LDLc, apolipoprotein B and triglycerides.
The Phase 3 MAESTRO-NAFLD-1 trial was a double-blind placebo-controlled trial with 969 patients with presence of three key risk factors namely Metabolic Syndrome, a level of liver fibrosis (measured by FibroScan) consistent with a range of stages of liver fibrosis, and >=8% liver fat (measured by MRIPDFF).
Resmetirom (MGL-3196), the Company’s lead product candidate, is a first-in-class, orally administered, small-molecule, liver-directed, thyroid hormone receptor (THR) β-selective agonist.
Paul Friedman, M.D., Chief Executive Officer of Madrigal commented,
“These positive results from the first of our two Phase 3 MAESTRO trials support our conviction that resmetirom has the potential to be the first medication approved for the treatment of patients with NASH and liver fibrosis. The blinded, placebo-controlled data from MAESTRO-NAFLD-1 reinforce previous positive Phase 2 and open-label Phase 3 safety findings in a much larger population of patients followed for 56 weeks. Rigorous safety evaluation is critical in NASH drug development because of the large numbers of patients with NASH that could be treated with a new medication once FDA approved. The large safety database we are generating through the MAESTRO trials supports our regulatory strategy under Subpart H and reflects our commitment to providing the data necessary for overall benefit-risk assessment,”
Most common adverse events reported include generally mild diarrhea or increased stool frequency at the start of the therapy. The Company intends to continue data generation from the MAESTRO-NAFLD-1 study and will present information related to safety, additional biomarkers and non-invasive measures of liver fibrosis before presenting or publishing the data at any major conference.
The Company is also engaged in a Phase 2 registration program for treatment of NASH (Non-alcoholic steatohepatitis). MAESTRO-NASH is a Phase 3 trial of resmetirom in liver biopsy confirmed NASH. The study had achieved the target enrollment of 900 patients with biopsy-proven NASH (fibrosis stage 2 or 3, at least 450 fibrosis stage 3), who will receive resmetirom 80 mg once a day, 100 mg once a day, or placebo. The primary surrogate endpoint of the study is NASH resolution, with at least a 2-point reduction in NAS (NASH Activity Score) and no worsening of Fibrosis. Secondary endpoints include liver fibrosis reduction of at least one stage, with no worsening of NASH on liver biopsy, and lowering of LDL-cholesterol.
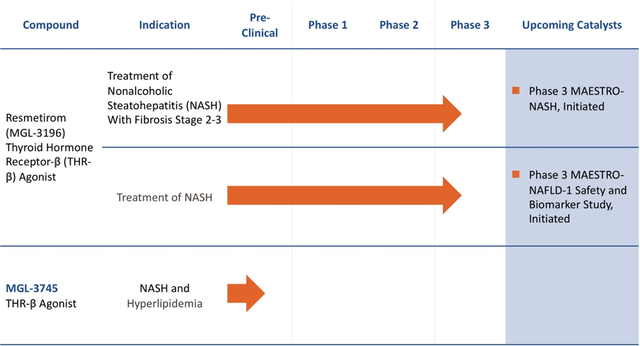
Image Source: Company
Madrigal’s pipeline has another candidate in preclinical development namely MGL-3745 a THR-B agonist intended for the treatment of NASH and Hyperlipidemia.
Weakness
In February 2018, the FDA issued a set of guidelines for developing drugs intended for the treatment of NASH with liver fibrosis. The guidance seeks to address the challenges associated with drug development in the area such as lack of criterion for identifying patients with nonalcoholic fatty liver (NAFL) that will progress to NASH. Therefore, the guidelines call for developing treatments for noncirrhotic NASH with liver fibrosis until better diagnosis is available to identify patients at the risk of progression.
The guidelines also lay down recommendations for trial design as well as endpoint selection that enable approval of treatments. The final call for approval of any treatment is dependent on how the FDA perceives the risk-benefit of the candidate and will take into consideration many critical aspects.
The Company is yet to release data on other key parameters such as key liver function tests, fibrosis markers, thyroid hormone tests and weight loss that will play an important role in determining the candidate’s efficacy.
Madrigal is expected to release the Fibrosis data in 2022, which will reveal the most crucial liver biopsy data that will measure fibrosis. The results from this study will be the deciding factor for its approval and are pertinent to scoring an approval from the FDA.
The Company had cash and cash equivalents of $299.1 million at the end of September 30,2021, which included amount from a at-the-market (ATM) sales agreement. Madrigal sold 1,347,290 shares for a total consideration of $151.2 million. However, the Company may look at raising additional capital to fund the MAESTRO-NASH trials and will possibly use the opportunity of positive data from the MAESTRO-NAFLD-1 to raise cash.
The liver fat reduction observed in MAESTRO-NAFLD-1 may not translate into improved fibrotic data and could raise questions about the Company’s approach to use a THR beta agonist to target fibrosus in NASH.
The Company’s competitor Intercept, which is developing Ocaliva for the treatment of NASH, is scheduled to meet the FDA in the first half of 2022 for a potential refiling of its application for Ocaliva. There is a possibility that the FDA may approve the candidate, making it the first drug to be approved for the indication and have an impact on its addressable market.
Opportunity
Nonalcoholic Steatohepatitis (NASH) is a form of Non-Alcoholic Fatty Liver Disease (NAFLD) and is characterized by the buildup of FAT in the liver. This condition is marked by hepatitis, inflammation, cell damage and fat deposits in the liver. These fat deposits can cause fibrosis of the liver, in turn leading to liver cancer or cirrhosis.
According to an estimate only 20 percent of the people suffering from NAFLD have NASH, while the rest only have a simple fatty liver. While nearly 40 percent in the U.S are afflicted with NAFLD, around 3 to 4 percent have NASH. The American Liver foundation estimates that over 100 million people suffer from NAFLD in the U.S alone.
Though the exact cause of the disease is still unknown, NASH often develops from underlying conditions such as obesity and type 2 diabetes, and can affect people of any age. Individuals who have insulin resistance, high triglyceride levels or abnormal cholesterol levels, hypertension and uncontrolled blood glucose are at an increased risk of developing this condition. NASH also increases the chances of developing cardiovascular anomalies and can lead to death from liver-related causes.
The diagnosis usually involves blood tests, use of imaging techniques such as ultrasound, CT scans and MRI, and a liver biopsy. Losing weight through a healthy diet and exercise can help reduce the fat in the liver and is usually the recommended course of action. Currently there are no approved therapies for this condition, with treatment limited to alleviating the symptoms of the condition.
According to a report by Reports and Data, the Global NASH market will be worth over USD 13.38 Billion by 2026. The healthcare costs associated with this disease are likely to reach USD 18 billion by 2030 from USD 5 billion now, if the disease is left untreated.
Threat
The Company’s pipeline is currently centered around one candidate – Resmetirom (MGL-3196). Clinical Trials are fraught with risk and uncertainty. However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to muster a regulatory approval. The success of its clinical trials will help the Company advance its pipeline but it should also be prepared to face any setbacks, in case its ongoing trials fail to meet their endpoints.
The clinical development of potential drug candidates in NASH has been extremely challenging, with many of them failing to show any significant improvement or benefit in larger clinical trials, in spite of demonstrating promising results in pre-clinical or early-clinical development. In July 2020, Genfit’s elafibranor was unable to achieve the primary endpoint of resolving NASH without worsening fibrosis scarring compared to placebo as well as its secondary endpoint in the Phase 3 RESOLVE-IT trial. The candidate joins a long list of similar failures in the NASH landscape which have failed to demonstrate significant impact in pivotal trials, tumbling at the last hurdle.
Ocaliva (obeticholic acid), an Farnesoid X receptor (FXR) agonist, being developed by Intercept that showed at least a one-stage improvement in fibrosis but failed to prevent the worsening of fibrosis, failed to win an FDA approval in June 2020.
Madrigal Pharmaceuticals is hopeful that its differentiated approach may finally prove to be the breakthrough that the NASH treatment landscape needs.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.madrigalpharma.com/wp-content/uploads/2022/01/MAESTRO-NAFLD-1-Topline-Release-FINAL.pdf
https://www.madrigalpharma.com/wp-content/uploads/2022/01/MAESTRO-NAFLD-1-Topline-Release-FINAL.pdf
https://www.madrigalpharma.com/ourapproach/pipeline/
https://www.fiercebiotech.com/special-report/4-elafibranor-2020-s-top-10-clinical-trial-flops
https://www.biospace.com/article/intercept-pharma-cmo-departs-company-three-months-after-ceo-leaves/





No Comments