
22 Jun Acadia Pharmaceuticals: What’s Next after the Negative FDA AdCom?
Acadia Pharmaceuticals, Inc. (NASDAQ: ACAD), a biopharmaceutical company developing small molecule drugs for treating central nervous system disorders, faced a setback at the Psychopharmacologic Drugs Advisory Committee (PDAC) meeting for pimavanserin, a drug intended to treat hallucinations and delusions associated with Alzheimer’s disease psychosis. The U.S. Food and Drug Administration (FDA)’s PDAC voted 9 to 3 against the drug as it felt the evidence did not support pimavanserin as an effective treatment for hallucinations and delusions in AD.
Steve Davis, Chief Executive Officer, commented on the development,
“We are disappointed with the outcome of today’s vote. We will continue to work closely with the FDA as it reviews the totality of our efficacy and safety data to enable a full assessment of pimavanserin’s benefit-risk in patients with ADP. We continue to believe there is substantial evidence across multiple independent clinical studies and endpoints that support the efficacy of pimavanserin in ADP. There are no FDA-approved treatments for this critical public health need and off-label use of multi-receptor acting antipsychotics has demonstrated poor patient outcomes, including worsening of cognition and motor function.”
The FDA had asked for the advice of the PDAC but is not bound by the recommendations, though it takes into account the committee’s advice for any drug applications. The FDA has set a target action date of August 4, 2022. Pimavanserin is a selective serotonin inverse agonist and antagonist preferentially targeting 5-HT2A receptors that play a selective serotonin inverse agonist and antagonist preferentially targeting 5-HT2A receptors that play a vital role in neuropsychiatric disorders.
NUPLAZID, the trade name for Pimavanserin, intended for treating hallucinations and delusions associated with Parkinson’s disease psychosis (PDP), was first approved by the FDA in 2016.
Acadia Pharmaceuticals, Inc. (NASDAQ: ACAD)
Market Cap: $2.1B; Current Share Price: 13.01 USD
Data by YCharts
We take a holistic look at the Company through a SWOT analysis below:
Company
The Company was founded in 1993 as Receptor technologies and leveraged its high-throughput screening offering based on its proprietary functional genomics platform, R-SAT. The name was changed to Acadia Pharmaceuticals in 1997 and refocused its priorities on building a portfolio of product candidates, besides entering into drug discovery and development collaboration with Allergan. In 1999, the Company’s team of scientists initiated a project to discover new non-dopaminergic antipsychotic agents selectively acting as inverse agonists on the 5-HT2A receptor, which led to the synthesis of Pimavanserin in 2001.
The Company received a breakthrough designation for NUPLAZID® (pimavanserin) from the FDA in 2014 for treating hallucinations and delusions associated with Parkinson’s disease psychosis. Consequently, in 2015 Acadia was granted a priority review for its New Drug Application (NDA) for NUPLAZID® (pimavanserin). The drug scored an FDA approval in 2016.
Pimavanserin has also received a Breakthrough Therapy Designation for treating hallucinations and delusions associated with dementia-related psychosis.
The Company is pursuing strategic acquisitions, collaborations, and partnerships, such as the acquisition of CerSci Therapeutics, which added a novel pain program to the Company’s portfolio. In 2018, the Company signed an exclusive North American license agreement for trofinetide, intended for treating Rett Syndrome, with Neuren Pharmaceuticals. In 2020, the Companies announced the grant of a rare pediatric disease designation for trofinetide. Acadia has also entered into an exclusive licensing agreement and research collaboration with Vanderbilt University to develop drug candidates targeting the muscarinic M1 receptor to treat a wide range of central nervous system disorders. Most recently, the company has entered into a collaboration with Stoke therapeutics to develop RNA-based treatments for severe and rare genetic neurodevelopmental diseases.
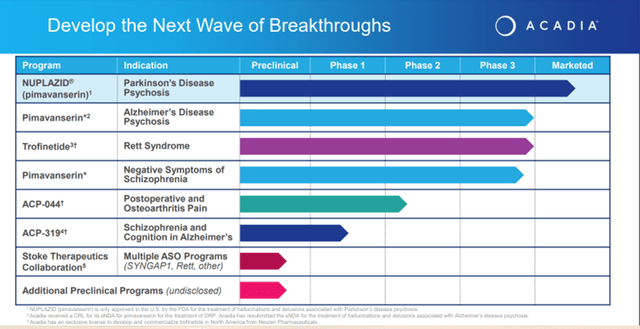
Image Source: Company
The Company’s pipeline consists of Pimavanserin, for which the Company resubmitted a supplemental NDA in February 2022 to treat hallucinations and delusions associated with Alzheimer’s disease psychosis (ADP). The resubmission was in response to the FDA’s Complete Response Letter (CRL) issued in April 2021 about the sNDA for the proposed indication for pimavanserin for treating dementia-related psychosis.
Upcoming catalysts for the Company include the top-line results from the Phase 3 ADVANCE study evaluating Pimavanserin in treating negative symptoms of schizophrenia that is anticipated in 2023; the submission of an NDA for trofinetide for the treatment of Rett Syndrome in mid-2022 and the results from a Phase 2 study in chronic pain model of osteoarthritis to be completed in the first half of 2023.
Weakness
The FDA’s Psychopharmacologic Drugs Advisory Committee (AdComm) had 12 members, of which three voted ‘yes’ and nine voted ‘no’ in response to the question “Does available evidence support a conclusion that pimavanserin is effective for the treatment of hallucinations and delusions in the AD [psychosis] population?”. Some of the committee members want an additional randomized controlled trial, including a withdrawal trial. In addition, the committee wished for a better representation of ethnicity and race and a long-term efficacy assessment.
The committee members who voted yes felt that there was a sizable unmet patient need, and the fact that the drug was already approved for a similar indication in Parkinson’s alleviated safety concerns.
Though the committee’s decision is not binding, the FDA usually considers the committee’s advice in drug applications. There is a possibility that the FDA may not approve the drug as its PDUFA date of August 4, 2022 approaches.
The FDA earlier served the Company a complete response letter in April 2021 for pimavanserin in the use of treatment for hallucinations and delusions associated with dementia-related psychosis. The FDA did not feel that the drug could be approved in its current form, citing a lack of statistical evidence in some subgroups of dementia and a lack of sufficient numbers in less common dementia subtypes as the reason for non-approval. Moreover, the FDA also opined that the phase 2 AD psychosis study –019, included in the sNDA filing, was not adequate or well-controlled. Furthermore, the FDA noted that the study was carried out at a single center and did not have a type 1 error control of secondary endpoints, leading to deviations.
The latest setback will affect the chances of the label expansion for pimavanserin. Nuplazid is the only FDA-approved drug of the Company and brought in a revenue of $493 million; however, adding additional indications to its portfolio would mean higher revenues. However, the Company may face challenges in achieving its goal, considering the adverse decision from the Psychopharmacologic Drugs Advisory Committee (AdComm).
Opportunity
Alzheimer’s is the sixth-leading cause of death in the U.S. This disease causes irreversible degeneration of the brain cells. It affects a person’s cognitive and behavioral abilities, such as impaired judgment, deteriorating memory, and personality changes with the gradual loss of motor abilities and speech.
According to some compelling statistics by Alzheimer’s Association, over 5.8 million Americans are affected by this disorder. The numbers will likely reach 14 million by 2050, with a new diagnosis being made every 65 seconds. The healthcare cost of Alzheimer’s and other Dementia related ailments will be over $290 billion in 2019; these numbers will likely escalate to over $1.1 trillion by 2050.
Image Source: NIH
A report by transparency market research predicts that the global market for Alzheimer’s will reach US$6.4 billion by 2025, growing at a CAGR of 7.5%, from US$3.6 billion in 2017. The rise in the aging population, increased awareness about the benefits of combined therapy, and a favorable regulatory and government support environment are some factors that will contribute to the market’s growth. The economic burden of the disease on not only the patients but also caregivers will impede the market. The prohibitive cost of expensive treatment, especially in developing nations, is another obstacle to growth.
Alzheimer’s is one illness where innumerable attempts have been made to find a cure with no success. According to a report released by Pharmaceutical Research and Manufacturers of America (PhRMA), from 1998 to 2017, there have been about 146 failed shots at developing drugs for Alzheimer’s disease. However, recent technological advancements and the discovery of new molecules and combination therapies offer renewed hope to those suffering from this illness.
In June 2021, Biogen’s ADUHELM™ (aducanumab-ava) scored approval from the U.S. Food and Drug Administration (FDA) to become the first drug approved after 2003 for the treatment of Alzheimer’s disease, which specifically addresses the underlying cause of the disease, by removing amyloid-beta from the brains of those afflicted with the condition.
According to a study, over 50% of patients with Alzheimer’s disease suffer from psychotic symptoms, delusions, and hallucinations. The condition manifests as delusions of persecution, abandonment, and impaired reality. Psychosis in Alzheimer’s disease denotes a more severe phenotype and is characterized by a more rapid cognitive decline even before the onset of psychosis. Moreover, individuals with psychosis are more likely to be agitated and aggressive, have a more significant functional impairment, and cause greater distress to family and caregivers.
Furthermore, pharmaceutical therapies for psychosis in AD have limited efficacy and can increase the risk of short-term mortality.
Threats
The Company’s pipeline has multiple candidates being evaluated in diverse indications. The other indications that the candidate is being assessed are either in Phase 2 / 3 trial stage. Clinical Trials are fraught with risk and uncertainty. There is a possibility that the candidates in the Company’s developmental pipeline may not be able to meet their clinical endpoints in trials.
The Company may fail to receive regulatory approval for any of the other candidates, resulting in a setback for the other candidates in the pipeline.
However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to meet endpoints in any of its ongoing trials. The success of its clinical trials will allow the Company to advance its pipeline, but it should also be prepared to face any setbacks in case its ongoing attempts fail to meet its endpoints.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.alz.org/alzheimers-dementia/facts-figures
https://www.transparencymarketresearch.com/pressrelease/alzheimers-drugs-market.htm
http://phrma-docs.phrma.org/files/dmfile/AlzheimersSetbacksSteppingStones_FINAL_digital.pdf





No Comments