
10 Nov After a Setback, What’s Next for Deciphera Pharmaceuticals?
Deciphera Pharmaceuticals, Inc. (NASDAQ: DCPH), a commercial-stage biopharmaceutical focused on creating novel therapies for treatment of cancer, announced that a crucial phase 3 trial of QINLOCK in patients with gastrointestinal stromal tumor (GIST) previously treated with imatinib did not meet the primary endpoint of improved progression-free survival (PFS) compared with the standard of care sunitinib.
The study named INTRIGUE was a randomized, global, multicentre, open-label study in which 453 patients were randomized 1:1 to receive either QINLOCK 150 mg once daily or sunitinib 50 mg once daily for four weeks followed by two weeks without sunitinib. QINLCOK did not achieve the primary efficacy endpoint of progression-free survival (PFS) as measured by a modified Response Evaluation Criteria in Solid Tumors (RECIST).
Steve Hoerter, President and Chief Executive Officer of Deciphera,
“While we are disappointed with these results, which we learned yesterday, we believe this was a robust, well-designed, and well-executed study. The full results from the INTRIGUE Phase 3 clinical study are expected to be presented at an upcoming medical meeting.”
Deciphera is a commercial-stage biopharmaceutical company that is leveraging its proprietary switch-control kinase inhibitor drug delivery platform to create novel treatments aimed at the inhibition of protein kinases. The Company has an approved drug in the market, namely Ripretinib, which is a broad-spectrum inhibitor of KIT and PGDFRA. The Company’s pipeline consists of Vimseltinib, an orally administered inhibitor of CSF1R that is currently being evaluated in a phase 1 / 2 trial; Rebastinib an inhibitor of TIE2, currently being evaluated in a Phase 1B/2 in Solid Tumors in Combination with Paclitaxel and DCC – 3116, an inhibitor of ULK, undergoing a Phase 2 trial.
Deciphera Pharmaceuticals (NASDAQ: DCPH)
Market Cap: $566.09M; Current Share Price: 9.68 USD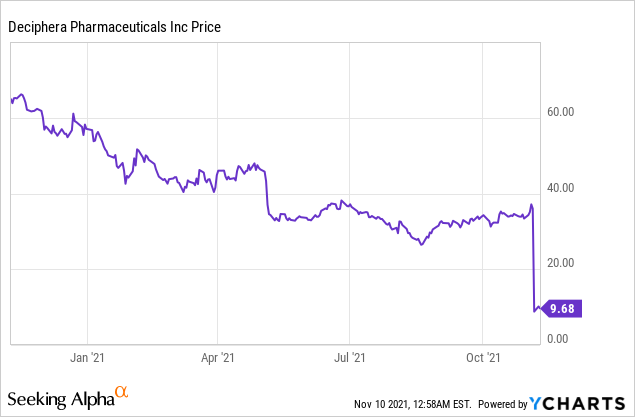
Data by YCharts
Strengths
Deciphera is leveraging a proprietary drug discovery platform to develop novel therapeutics for the treatment of cancer. The Company is using its expertise in Kinase biology to design kinase inhibitors that have the potential to target the switch pocket region to deliver therapeutic solutions that can improve patient outcomes.
Image Source: Company
Kinases play an important role in regulating cellular functions and any dysregulation can result in cancer, inflammatory and autoimmune diseases. The Company’s differentiated approach involves using kinase inhibitors that interact at a molecular level, unlike other kinase inhibitors, thereby demonstrating a more durable response rate.
The Company’s pipeline consists of Vimseltinib, an orally administered switch-control kinase inhibitor of the CSF1R switch pocket that is currently being evaluated in a Phase 1 /2 in Tenosynovial Giant Cell Tumor (TGCT); Rebastinib, an orally administered switch-control kinase inhibitor of the TEK tyrosine kinase, or TIE2 being studied in a Phase 1b /2 in Solid Tumors in Combination with Paclitaxel and DCC-3116 an inhibitor of ULK, currently undergoing Phase 1 trial in RAS/RAF Mutant Cancers in Combination with Trametinib.
Weakness
The sales figures from Qinlock have left much to be desired since its launch in 2020. Presently, the drug is only a fourth-line treatment for patients previously treated with three other lines of treatment. In addition, GIST is a rare form of cancer and hence a therapeutic targeting it has a limited market for expansion to begin with.
The Company was hopeful that positive Phase 3 results would help it push the drug to the second-line setting, which would mean that it could tap a potential $ 1 billion market. However, the failed phase 3 clinical trial will now put a spoke in the wheel of its plans. As per its Q2,2021 financial results, the Company recorded $22.0 million in QINLOCK net product revenue, including $20.7 million in U.S. and $1.3 million in ex-U.S. sales.
The Company’s focus on cancer and its pipeline could make it a potential target for a larger pharmaceutical company. However, setbacks in a clinical trial may raise questions about the company’s approach, drug’s efficacy and its future prospects.
Opportunity
Gastrointestinal stromal tumors (GISTs) are a rare form of cancer that originates in special cells called interstitial cells of Cajal (ICCs), in the walls of the gastrointestinal (GI) tract. The gastrointestinal tract also known as the digestive tract plays a crucial role in processing food and getting rid of solid waste from the body.
GIST’s can start in the stomach or in any part of the GI tract or in some cases even outside the GI tract in the omentum or peritoneum. The tumor can be localized or spread to other parts of the body based on factors such as size, location and mitotic rate. Some of the risk factors for developing GIST include age and genetic inheritance of certain genes such as PDGFRA gene or SDH (succinate dehydrogenase) genes.
GIST’s are different from other types of stomach cancers such as adenocarcinomas, squamous cell carcinomas or neuroendocrine tumors (NETs) as they start in different cells and have to be treated differently. The current treatment options include surgery, targeted drug therapy, ablation and embolization, chemotherapy and radiation therapy.
According to a report by persistence market research, the global Gastrointestinal stromal tumors (GISTs) market will be worth over $1.5 billion in 2026, growing at a CAGR of 6.6% from 2017. According to the WHO, GIST’s are the most common primary mesenchymal tumors of the gastrointestinal tract and are found in 14.5 per million, with a prevalence 129 per million, and 5000 to 6000 new diagnoses per year in the United States.
Threats
Clinical Trials are fraught with risk and uncertainty. There is a possibility that the candidates in the Company’s developmental pipeline may not be able to meet their clinical endpoints in trials. However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to meet endpoints in any of its ongoing trials. The success of its clinical trials will help the Company advance its pipeline but it should also be prepared to face any setbacks, in case its ongoing trials fail to meet their endpoints.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.cancer.org/cancer/gastrointestinal-stromal-tumor/treating.html
https://www.persistencemarketresearch.com/market-research/gastrointestinal-stromal-tumor-market.asp
https://www.pharmaceutical-technology.com/news/deciphera-zai-lab-ripretinib-licensing/





No Comments