
18 Jun Can Annovis Bio Deliver a Breakthrough in Alzheimer’s Disease?
Annovis Bio, Inc. (NYSE: ANVS) is a clinical-stage biotechnology Company that is developing a pipeline of drugs that target the treatment of Alzheimer’s and other neurodegenerative diseases through improvement of Axonal Transport. The Company is working on therapeutics for Alzheimer’s disease (AD), Parkinson’s disease (PD), Dementia and Alzheimer’s in Down Syndrome (AD-DS) (granted an orphan drug designation by the FDA).
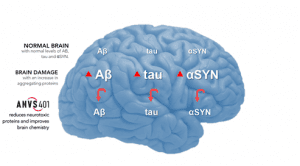
Image Source: Company
The Company is adopting a differentiated approach from other companies focusing on Amyloid Plaque and Aβ that hasn’t yielded any result so far, as evidenced by numerous failures in the clinical trials of drugs targeting the idea of sticky brain plaques causing Alzheimer’s disease. Annvois is concentrating on neurotoxic proteins that hamper axonal transport and cause a toxic cascade, which leads to reduced neurotransmitters and neurotrophic proteins, which in turn results in nerve cell death. The idea is to address the root of the problem in neurodegeneration, which is impaired axonal transport caused by increasing levels of neurotoxic proteins, as against an approach that targets plaques or lewy bodies that are the end stage of neurodegeneration.
In June 2021, Annovis announced preliminary data from a study initiated last year that has demonstrated the potential of ANVS401 to protect nerve cells from death resulting from an infection by gingipains, the virulence factors of Porphyromonas gingivalis (P. gingivalis). The Company intends to explore the possibility of the candidate being used in the treatment of neurological diseases caused due to bacterial and viral infections.
Annovis Bio, Inc. (NYSE: ANVS)
Market Cap: $ 625.60M; Current Share Price: 90.05 USD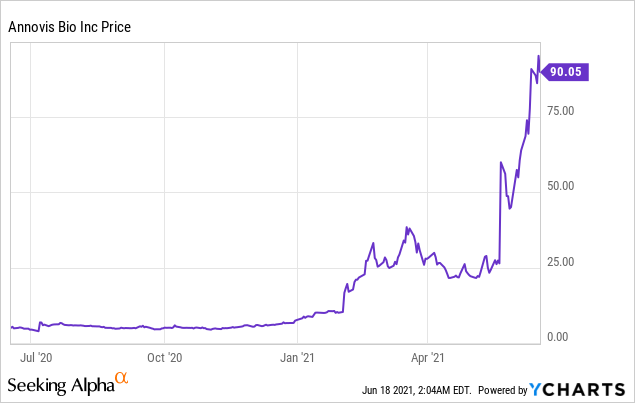
Data by YCharts
Industry
Alzheimer’s is the sixth leading cause of death in the U.S, a disease that causes irreversible degeneration of the brain cells and affects a person’s cognitive and behavioral abilities such as impaired judgment, deteriorating memory and personality changes with the gradual loss of motor abilities and speech.
According to some compelling statistics made available by Alzheimer’s Association over 5.8 million Americans are affected by this disorder and the numbers will most likely reach 14 million by 2050, with a new diagnosis being made every 65 seconds. The healthcare cost of Alzheimer’s and other Dementia related ailments will be over $290 billion in 2019, these numbers are likely to escalate further to over $1.1 trillion by 2050.
Image Source: NIH
A report by transparency market research predicts that the global market for Alzheimer’s will reach US$6.4 billion by 2025, growing at a CAGR of 7.5%, from US$3.6 billion in 2017. The rise in ageing population, increased awareness about the benefits of combined therapy, a favorable regulatory and government support environment are some of the factors that will contribute to the growth of the market. The economic burden of the disease on not only the patients but also caregivers will act as an impediment for the market. The prohibitive cost of expensive treatment especially in developing nations is another obstacle for growth.
Alzheimer’s is one illness, where innumerable attempts have been made to find a cure with no success. According to a report released by Pharmaceutical Research and Manufacturers of America (PhRMA) from 1998 to 2017 there have been about 146 failed shots at developing drugs for Alzheimer’s disease. However recent advancements in technology and the discovery of new molecules and combination therapies are offering renewed hope to those suffering from this illness.
Parkinson’s is the second most common age-related neurodegenerative disorder, characterized by muscle rigidity, bradykinesia, cognitive impairment etc. An estimated one million people in the USA are currently battling this disease, with over 60,000 new diagnoses each year. The cost of healthcare is a staggering $25 billion per year which includes treatment cost and lost income.
A report by market research engine projects a CAGR of 6% for the Parkinson’s disease treatment market, and expects it to reach US$ 6.0 Billion by 2024. A rise in geriatric population with growing incidences of Parkinson’s coupled with the emergence of novel combination therapies such as dopaminergic stimulation medicines, gene therapy and neural transplantation will help the market grow, while the prohibitive treatment costs and the introduction of generics may act as limiting factors.
Researchers have identified Alpha-synuclein (also α-synuclein) protein as the cause of this disorder though its role in brain regulation is unknown. Alpha-synuclein has been the focus of extensive research efforts to understand its role in Parkinson’s and as a prospective base for novel neuroprotective therapies.
Companies are increasingly exploring newer mechanisms of combating this illness and with emergence of novel technologies the focus has shifted to innovative treatment methods such as Gene therapy, peptide-based therapeutics, dopamine agonists and protein inhibitors.
Company
Annovis’s lead candidate ANVS401, is under clinical development for the treatment of AD-DS, AD and PD and has demonstrated the ability to inhibit neurotoxins and normalize axonal transport in preclinical studies. In addition, the candidate is able to reduce APP/Aβ, tau/phospho-tau and α-synuclein, which are responsible for causing inflammation and cell death. Additionally, ANVS401 was shown to normalize levels of APP, tau and αSYN in the cerebrospinal fluid (CSF), besides being well-tolerated and safe, in its third Phase 1 clinical study.

Image Source: Company
The Company plans to initially focus on AD-DS in Phase 3 and intends to use the orphan status to obtain human data for AD much faster than in a regular AD population. In studies related to trisomic DS animals and nerve cells, the candidate was able to restore and improve axonal transport, reduce APP, tau and phospho-tau and increased levels of BDNF (a neurotrophic factor), leading to restored memory and learning and returned the brain to homeostasis.
ANVS401 has also been evaluated in mice with Parkinson’s disease and treatment with the candidate has been able to lower aSYN in the gut and in the brain and fully normalizes colonic motility.
The Company’s pipeline also consists of ANVS405, an injectable drug for protecting the brain from TBI and/or stroke in cases of acute brain/head trauma. The development of the candidate is funded by the US Army and the Company intends to apply for further grants for its development. Furthermore, Annovis is also developing ANVS301, intended for the treatment of advanced AD and Dementia, which is currently undergoing a Phase 1 clinical trial being conducted and sponsored by the National Institutes of Health (NIH).
Annovis is currently engaged in a Phase 2a study in AD patients in collaboration with the Alzheimer’s Disease Cooperative Study (ADCS) and a Phase 2a study in AD and PD Patients.
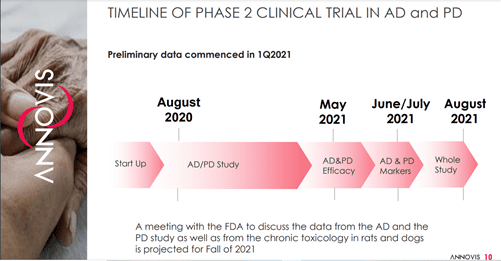
Image Source: Company
The Company has reported data from the AD & PD efficacy trial in May 2021 and will soon release the AD & PD marker data in June/July 2021, with the results from the complete study expected to be announced in August 2021. The results from the Phase 2a trial show statistically significant improvement in parameters such as cognition, motor function, WAIS coding and Inflammation in patients with AD and PD.
Annovis is also planning to meet the FDA in the fall of 2021 to discuss data from the AD and PD study along with data from chronic toxicology in rats and dogs.
Key Takeaways
- The efficacy data of Annovis is far more encouraging than that of Biogen’s Aduhelm (aducanumab), a drug that was recently approved by the FDA for treatment of Alzheimer’s despite questions raised about its efficacy. Preliminary data from its phase 2 study shows ANVS401 was able to improve cognition by almost 30 percent in just 25 days. Another important differentiating factor between these two drugs is that while aducanumab requires a monthly transfusion, ANVS401 is orally administered, which is far more convenient from a patient point of view, and may be a deciding factor if the drug receives approval from the FDA.
- Clinical Trials are fraught with risk and uncertainty. The Company has three candidates in the pipeline and is focused on developing a diverse pipeline of candidates which will help mitigate the risk in case of adverse results or the failure to meet endpoints in any of its ongoing trials of a particular candidate. The success of its clinical trials will help the Company advance its pipeline but it should also be prepared to face any setbacks, in case its ongoing trials fail to meet their endpoints.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.annovisbio.com/technologies
https://www.annovisbio.com/technologies
https://irpages2.eqs.com/websites/annovis/English/4310/news.html





No Comments