
28 Nov Aytu BioPharma: Leading the ADHD Drug Market
Aytu BioPharma, Inc. (NASDAQ: AYTU) is a pharmaceutical company focused on commercializing novel therapeutics. The Company is witnessing increasing market support for its attention deficit hyperactivity disorder (ADHD) Portfolio and Pediatric Portfolio and is thus focusing its resources on these rapidly growing products.
Aytu BioPharma, Inc. (NASDAQ: AYTU)
Market Cap: $14.03M; Current Share Price: 2.50 USD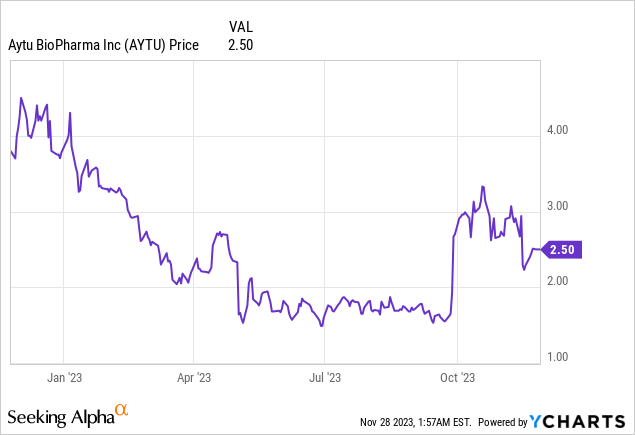
Data by YCharts
The Company and its Products
Aytu was initially incorporated as Rosewind Corporation on August 9, 2002, in the State of Colorado and re-incorporated as Aytu BioScience, Inc. in Delaware on June 8, 2015. Following the acquisition of Neos Therapeutics, Inc. in March 2021, the Company changed its name to Aytu BioPharma, Inc.

Image Source: Company
The Company currently manufactures its ADHD medications, Adzenys XR-ODT and Cotempla XR-ODT, in its Grand Prairie, Texas facility. In April 2023, AYtu received approval from the U.S. Food & Drug Administration (FDA) for the Adzenys Prior Approval Supplement (PAS), which enables the transfer of manufacturing of Adzenys to a third-party manufacturer. In June 2023, the Company submitted the Cotempla PAS to the FDA and expects to have a six-month review process for the Cotempla PAS.
AR101 (enzastaurin) is a development-stage asset being developed as an investigational treatment for Vascular Ehlers-Danlos Syndrome (VEDS), a rare connective tissue disorder for which no approved treatments exist. AR101 has received Orphan Drug Designation from both the FDA and the European Commission, thus making AR101 eligible for market exclusivity upon product approval. AR101 also received Fast Track Designation from the FDA, given the urgent, unmet need in VEDS. The Company expects the development of AR101 to advance once it can either fund development through operating cash flows or through an out-license or sale to a strategic partner.
The prescription Pediatric Portfolio includes Karbinal® ER, an extended-release carbinoxamine (antihistamine) suspension indicated to treat numerous allergic conditions for patients two years and above, and Poly-Vi-Flor® and Tri-Vi-Flor®, two complementary prescription fluoride-based multi-vitamin product lines containing combinations of fluoride and vitamins in liquid and chewable tablet form for infants and children with fluoride deficiency (Karbinal ER, Poly-Vi-Flor and Tri-Vi-Flor are collectively the “Pediatric Portfolio”). These products serve established pediatric markets and offer distinct clinical features and patient benefits.
Aytu commercializes its Rx Portfolio through an internal commercial organization that includes approximately forty sales territories for the ADHD Portfolio and about six for the Pediatric Portfolio.
The Aytu RxConnect™ patient support program operates through a network of approximately 1,000 pharmacies. It offers affordable, predictable co-pays and hassle-free availability to all commercially insured patients, regardless of their insurance plan.

Image Source: Company
It is important to note that the Company plans to wind down its Consumer Health segment soon to better concentrate on the Rx segment.
We’ll discuss the critical rationale for covering this Company.
- Supportive Market Trends
The market for ADHD drugs and fluoride supplements has shown increasing trends.
ADHD is one of the most common developmental disorders in children and often persists into adulthood. In 2022, CDA reported that 6 million children in the United States, ages 3 to 17, had previously received an ADHD diagnosis between 2016 and 2019, up 36% since 2003. During the same year, approximately 83.5 million prescriptions for ADHD medications were written in the United States and generated roughly $21.2 billion in sales.
Extended-release, or long-acting, dosage forms of stimulant medications are the standard of care for treating ADHD, making up approximately 43% of ADHD prescriptions. So far as ADHD drugs are concerned, Aytu has an advantage over its peers – its drug Adzenys XR-ODT is FDA-approved as bioequivalent to Adderall XR and is the first and only orally disintegrating tablet (ODT) extended-release amphetamine. ODTs have a rapid onset, are easy to take, and help caregivers prevent “cheeking” or patient non-use of ADHD medications.
Since 2022, numerous Adderall XR and Concerta generic manufacturers have reported ongoing, intermittent manufacturing delays contributing to supply shortages – this further underlines the increasing demand and importance for Aytu’s ADHD drugs.

Image Source: Company
Regarding fluoride market dynamics, the American Dental Association has stipulated that fluoride supplements can be prescribed for children ages six months to 16 years at high risk for tooth decay and whose primary drinking water contains low or no fluoride.
While most US drinking water is fluoridated, some major geographic areas lack it, including much of New Jersey and New York’s Long Island. Approximately 1 in 4 American children live in municipalities that do not fluoridate the water supply or in rural areas that rely on well water and do not receive recommended fluoride levels through fluoridation. In 2021, 9.5 million multi-vitamin prescriptions were written in the U.S. Of those prescriptions, multi-vitamins containing sodium fluoride accounted for 1.5 million total prescriptions.
Aytu offers Poly-Vi-Flor® and Tri-Vi-Flor® – two complementary prescription fluoride-based supplement product lines containing combinations of multiple vitamins and sodium fluoride in various oral formulations.
Thus, we find that Aytu is well-positioned to benefit from increasing market demand for ADHD drugs and fluoride supplements and should perform well in the future.
- Comprehensive growth strategy
Aytu employs a focused approach of in-licensing, acquiring, developing, and commercializing novel prescription therapeutics and consumer health products. As discussed, its primary focus is on commercializing innovative prescription products that address conditions frequently created or diagnosed in childhood, including ADHD.
The Company’s strategic priorities are to continue to increase revenues and enhance financial performance through operational and manufacturing efficiencies and portfolio prioritization. Specifically, it intends to:

Image Source: Company
Thus, Aytu has created a well-rounded growth strategy to derive maximum benefit from the increasing demand for its products, and this should help the Company confidently move forward.
- Financial performance
The Company has recorded strong revenue growth over the past three years driven by organic growth and strategic acquisitions driving a $100M+ annualized run rate.
For FY23 (Jun), total net revenue increased 11% to $107.4 million from $96.7 million in the year-ago period, while FY23 Rx Segment net revenue was $73.8 million, compared to $61.1 million last year, a growth of 21%. During this period, ADHD products grew 9% YoY, while Pediatric products grew 58% YoY.

Image Source: Company
FY23 Rx Segment Adjusted EBITDA was a positive $9.4 million. At the same time, Q4 2023 Rx Adjusted EBITDA was positive $8.3 million with an EBITDA margin of 35%.

Image Source: Company
For Q4 FY23, record prescription trends of up 32% across ADHD and pediatric portfolios drove revenue growth, and as of June 30, 2023, the Company had cash on hand of $23 million.
For Q1 FY24, total net revenue was $22.1 million, compared to $27.7 million. The change was primarily due to the planned wind-down of the Company’s Consumer Health segment and the associated decrease in Consumer Health revenue. ADHD products (Adzenys XR-ODT® and Cotempla XR-ODT®) net revenue increased 31% to $15.1 million, compared to $11.6 million in the year-ago quarter.
Total Adjusted EBITDA1 was $2.2 million in Q1 2024 compared to $1.7 million in the year-ago quarter, a 32% increase, while cash and cash equivalents were $20.0 million at September 30, 2023.
Risks
Despite the Company’s promising performance and outlook, it is subject to certain risks. Firstly, if the U.S. Food and Drug Administration (FDA) or other applicable regulatory authorities approve generic or similar products that compete with Aytu’s commercial prescription products, or if the FDA or other relevant regulatory authorities change or create new pathways that may expedite approval of such products, it could decrease expected sales of the Company’s commercial prescription products.
Secondly, if Aytu cannot protect intellectual property rights or if intellectual property rights are inadequate to protect its technology, its commercial prescription products, or other product candidates, competitors could develop and commercialize similar technology, and Aytu’s competitive position could be harmed.
Finally, the Company’s efforts to expand and transform its business may require significant investments. It may be unsuccessful, or it may have difficulties integrating acquired businesses, and as a result, the business, results of operations, and financial condition may be materially adversely affected.
Conclusion
The market for Aytu’s ADHD and pediatric drugs is increasing, and the Company has a well-formed strategy to benefit from this phenomenon. Aytu also has a significant advantage over its competitors due to its drug Adzenys XR-ODT, which is the first and only orally disintegrating tablet (ODT) extended-release amphetamine. Finally, the Company’s Rx Segment has shown substantial growth over the last three years, indicating that Aytu’s efforts and strategies are showing promising results in the market.
Nevertheless, there is always the possibility that Aytu may lose its competitive edge due to changes in FDA regulations or the inability to protect intellectual property rights. In such circumstances, the Company’s prospects would be significantly hampered. Hence, all investors must proceed with caution.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
Reference:
https://www.sec.gov/ix?doc=/Archives/edgar/data/1385818/000155837023016393/aytu-20230630x10k.htm
https://www.sec.gov/ix?doc=/Archives/edgar/data/1385818/000155837023019015/aytu-20230930x10q.htm
https://feeds.issuerdirect.com/news-release.html?newsid=4858919631609309
https://feeds.issuerdirect.com/news-release.html?newsid=6680535930369308





Sorry, the comment form is closed at this time.