
25 Jul Key Catalysts Make Arcutis a Must Watch!
Arcutis Biotherapeutics, Inc. (NASDAQ: ARQT), a biopharmaceutical company developing novel treatments for dermatological diseases, announced the acceptance of the New Drug Submission (NDS) for roflumilast cream 0.3% (ARQ-151) for the treatment of plaque psoriasis in adults and adolescents, filed by Arcutis Canada Inc., a subsidiary of Arcutis Biotherapeutics, Inc.
A once-daily topical formulation of roflumilast, the cream acts as an inhibitor of phosphodiesterase type 4 (PDE4). The submission is supported by clinical trial results from a pivotal Phase 3 program and two long-term open-label studies. DERMIS 1 and DERMIS 2 (Trials of PDE4 inhibition with Roflumilast for the Management of plaque PsoriasIS” One and Two).
Arcutis Biotherapeutics, Inc. (NASDAQ: ARQT)
Market Cap: $1.11B; Current Share Price: $21.52
Data by YCharts
We take a holistic look at the Company through SWOT analysis.
Strength
The Company’s lead drug candidate Topical Roflumilast has demonstrated efficacy on par with steroid/vitamin D combinations despite being non-steroidal. Roflumilast can be used anywhere on the body and for extended periods. The Company does not expect a boxed warning for the drug upon approval as it has no evidence of application site reactions.
Arcutis is leveraging a unique dermatology drug development platform to create therapeutics that address significant unmet medical needs. The Company commissioned The Harris Poll to survey 507 U.S. adults (18+) with psoriasis, with 307 respondents having psoriasis in intertriginous areas at any time, to understand the emotional impact and challenges faced by them.
Besides Plaque Psoriasis, the Company is also developing candidates for treating Atopic Dermatitis, Seborrheic Dermatitis, Scalp Psoriasis, chronic hand eczema, Vitiligo, and Alopecia Areata.
The Company’s candidates act by inhibiting the JAK pathway, JAK1 in particular, which plays a crucial role in immune system function. This pathway’s targeting can potentially treat various inflammatory diseases like rheumatoid arthritis, psoriasis, Crohn’s disease, and atopic dermatitis, among others.
For instance, in the case of Alopecia Areata, the Company’s approach revolves around the use of a topical JAK Inhibitor, delivered directly to the site of inflammation, deep into the bulb of the hair follicle. Arcutis is developing ARQ-252 as an alternative topical formulation of ARQ-252. This topical JAK1 inhibitor can potentially address the treatment of various inflammatory dermatological diseases such as eczema and vitiligo, besides alopecia areata.
Arcutis is currently working on the formulation and preclinical activities and will advance the candidate into clinical trials upon completing the same. The candidate has the potential to become the only topical treatment for alopecia areata.
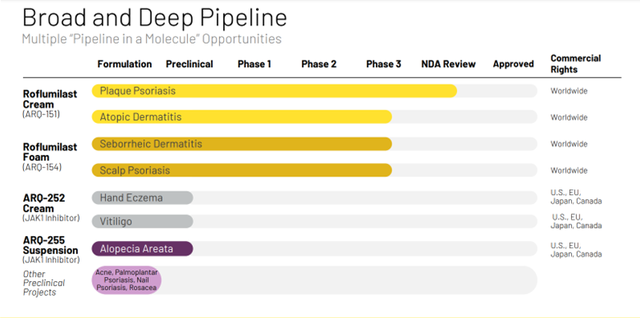
Image Source: Company
ARQ-252 offers more potency and high selectivity against JAK1 as compared to JAK2, which makes it much safer than other topical JAK inhibitors. The Company’s pipeline consists of Roflumilast Cream (ARQ-151) being evaluated in Plaque Psoriasis, Atopic Dermatitis; Roflumilast Foam (ARQ-154) being studied in Seborrheic Dermatitis and Scalp Psoriasis and preclinical programs in Hand Eczema, Vitiligo and Alopecia Areata.
The Company has entered into a licensing agreement with AstraZeneca (NASDAQ: AZN) for exclusive worldwide rights to the topical dermatological use of roflumilast, a small molecule inhibitor of phosphodiesterase type 4 (PDE4) for the treatment of chronic plaque psoriasis and atopic dermatitis. The agreement covers exclusive worldwide rights to all topical dermatological uses of roflumilast, including rights to any commercialized products.
In December 2019, Arcutis exercised its option under a 2018 licensing agreement with Jiangsu Hengrui Medicine Co., Ltd. of China for the active pharmaceutical ingredient in ARQ-252 for use in the United States, Canada, Europe, and Japan.
In January 2021, Arcutis initiated a Phase 3 trial in atopic dermatitis named INTEGUMENT-1 and INTEGUMENT-2 and were likely to announce the results from the study in 2023.
The Company has a $225M non-dilutive loan facility, of which $75 million was drawn at the close of Q4. Furthermore, $125 million can be availed at the time of FDA approval, with a further $25 million available to achieve revenue milestones. The company has availed of the loan facility at an attractive cost of capital and with minimal covenants. The Company believes the financing is sufficient to provide a cash runway into 2024.
Weakness
In July 2021, the Company had to terminate a Phase 2a clinical trial of its vitiligo candidate as the data suggested that the topical formulation may not be able to get enough active targets in the skin. The trial was initiated in March 2021 with 500 subjects to study the topical JAK1 inhibitor ARQ-252. However, the Company faced a setback as the Phase 2 study of ARQ-252 failed in chronic hand eczema as none of the arms were better than the control arm. The results from the failed study prompted the Company to shelve the vitiligo trial instead of waiting for topline data in H2,2023.
The decision to reformulate the drug and re-enter the clinical trials has set the Company back as competition marches ahead. Incyte’s Opzelura™ (ruxolitinib) cream for the short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis (AD) scored FDA approval in September 2021, making it the first and only topical Janus kinase (JAK) inhibitor approved in the United States. Leo Pharma initiated a Phase 3 trial of delgocitinib cream in adult patients with moderate-to-severe chronic hand eczema (CHE).
Opportunity
Psoriasis is an autoimmune skin disorder characterized by a rash accompanied by itchy scaly patches on certain parts of the body like the knees, trunk, scalp, and elbows. It is a chronic condition that is painful and disruptive to sleep and normal functioning. The condition can flare up due to triggers like medication for high blood pressure and malaria, and infections such as strep throat or skin conditions, cold/dry weather, sunburn, and bug bites. There is no cure for the disease, with treatment focussing on alleviating the symptoms and lifestyle changes to cope with the condition.
The disease is categorized into different categories based on the signs and symptoms. The most common form of psoriasis is plaque, which causes itchy plaques with scales on the knees, elbows, scalp, and back. The skin changes color due to post-inflammatory hyperpigmentation and turns brown or black. The disease can also affect nails leading to nail psoriasis causing abnormal nail growth or severe damage to the nails. Other psoriasis includes Guttate Psoriasis, Inverse Psoriasis, Pustular Psoriasis, and Erythrodermic psoriasis.
According to a report by Fortune Business Insights, the global psoriasis treatment market is expected to reach $ 47.24 billion by 2029, growing at a CAGR of 8.7% from $26.37 billion in 2022. The growth in the market will be driven by an aging population, significant investment in research and development, and government and regulatory support.
According to information from the National Psoriasis Foundation, more than 8 million people in the U.S currently have Psoriasis. There are 125 million people affected by this condition worldwide.
Threats
Clinical Trials are fraught with risk and uncertainty. There is a possibility that the candidates in the Company’s developmental pipeline may not be able to meet their clinical endpoints in trials.
The Company may fail to receive regulatory approval for any other candidates, resulting in a setback for the other candidates in the pipeline.
However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to meet endpoints in any of its ongoing trials. The success of its clinical trials will allow the Company to advance its pipeline, but it should also be prepared to face any setbacks in case its ongoing attempts fail to meet its endpoints.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.fortunebusinessinsights.com/industry-reports/psoriasis-treatment-market-100600
https://www.psoriasis.org/psoriasis-statistics/
https://www.arcutis.com/about/strategic-collaborators/
https://investors.arcutis.com/static-files/893fa0b0-3162-4d4d-96d3-a5b6974e4ba4
https://finance.yahoo.com/news/arcutis-biotherapeutics-reaches-regulatory-milestone-120500497.html





No Comments