
26 May Siga’s Smallpox Win is No Monkey Business!
On May 19, 2022, The Siga Technologies, Inc. (NASDAQ: SIGA) announced the approval of its intravenous (IV) formulation of TPOXX® (tecovirimat) by the U.S. Food and Drug Administration (FDA). The drug is intended to treat smallpox, and the approval offers a much-needed alternative to those individuals who cannot consume the pill orally. The oral formulation of the TPOXX is already approved for use in the US, Canada, and Europe, with the European approval also covering the treatment of monkeypox, cowpox, and complications from immunization with vaccinia. The drug was recently mentioned in the U.S. president’s budget request as being used to treat a patient with monkeypox.
Siga Technologies, Inc. (NASDAQ: SIGA)
Market Cap: $637.18M; Current Share Price: 8.80 USD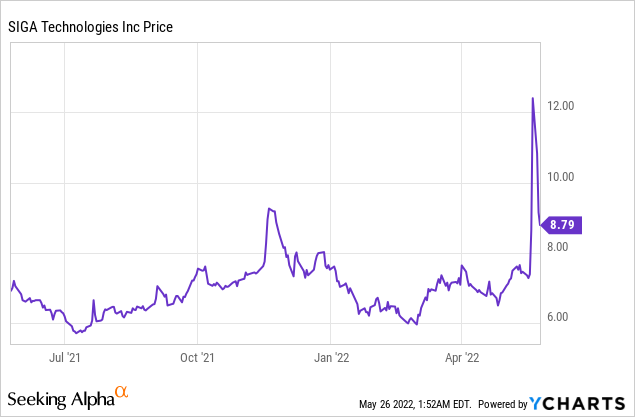
Data by YCharts
Commenting on the approval, Dr. Dennis Hruby, CSO of SIGA, stated,
“We are grateful to the FDA for their work leading to approval of IV TPOXX, which will provide access to a broader patient population. We are also appreciative to our colleagues at BARDA who have been working with us for many years to include oral and IV TPOXX in U.S. preparedness efforts and look forward to continuing to work with them on our liquid pediatric formulation.”
TPOXX was initially approved on July 13, 2018, as an oral treatment of smallpox, followed by an approval by Health Canada in December 2021. The European Medicines Agency (EMA) approved Tecovirimat (TPOXX) in January 2022 with the broader label.
Strength
SIGA is a commercial-stage pharmaceutical company focused on addressing the significant unmet need in the health security market, including developing medical countermeasures to combat biological, radiological, chemical, and nuclear (CBRN) threats and emerging infectious diseases.
The Company’s first FDA-approved product is oral TPOXX® (tecovirimat), approved in July 2018 to treat smallpox. TPOXX, also known as tecovirimat and ST-246®, is an orally administered and IV formulation small-molecule drug primarily intended to treat human smallpox caused by the variola virus. The drug works by inhibiting the viral maturation of the variola virus and preventing the formation of a secondary viral envelope, thereby arresting the spread of the virus.
On May 12, 2022, the Company announced the award of a contract by the U.S. Department of Defence (DoD) to procure up to approximately $7.5 million of oral TPOXX, of which $3.6 million of oral TPOXX is to be delivered in 2022. The DOD holds an option for the sole delivery of the rest. SIGA is actively collaborating with the DoD’s Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense (JPEO-CBRND) to develop a Post Exposure Prophylaxis (PEP) indication for TPOXX that comes under the purview of a separate contract worth $26 million. Consequently, TPOXX is now eligible for an Expanded Access Protocol for TPOXX and can be used for Post Exposure Prophylaxis (PEP) indications in certain DoD-affiliated personnel, pending FDA approval.
The Company had initially signed a contract with Biomedical Advanced Research and Development Authority (BARDA) in September 2018 to procure and develop both oral and intravenous formulations of TPOXX. The value of the contract is over $600 million if BARDA exercises all options. SIGA has delivered nearly $240 million of TPOXX® as part of the contract and another $460 million of oral TPOXX® under a contract awarded in 2011 to fill the requirements of the strategic stockpile.
The Company has built strong relationships with key health security constituencies in the U.S government, including organizations such as the Biodefense Advanced Research Development Authority (BARDA), the National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH), the Defense Threat Reduction Agency (DTRA), U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), and the Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRN).
Furthermore, the Company has established a robust network of over 20 partners encompassing numerous verticals such as discovery, pre-clinical, clinical, manufacturing, and supply chain that led to the development of TPOXX® and the delivery of $200 million of courses to the Strategic National Stockpile. The Company adopted a specialized approach based on the FDA’s animal rule for obtaining regulatory approval for smallpox, a disease that no longer exists in the human population.
Moreover, Siga’s highly externalized cost structure helps minimize fixed costs and offers scalability. The Company intends to use its expertise, infrastructure, and network to advance TPOXX in additional health security opportunities.

Image Source: Company
The Company’s pipeline consists of a liquid suspension pediatric formulation for patients who weigh less than 13 kgs and are not covered by the approved label indication for oral TPOXX. BARDA funds the development program, and the Company has generated formulation prototypes that will undergo clinical evaluation. In addition, SIGA is also developing a second mechanism of action for smallpox small-molecule antiviral drugs that has demonstrated efficacy in animal models.
Siga is working on testing the efficacy of TPOXX in cases of Monkeypox, which has shown antiviral activity against a variety of poxviruses, including Monkeypox. The drug was used for compassionate use for a patient suffering from Monkeypox at the Liverpool University Hospitals NHS Foundation Trust in the United Kingdom (UK). The Company has also entered into a collaboration with Oxford University to treat cases of Monkeypox under an expanded access protocol in the Central African Republic (CAR). The study is being sponsored by Oxford University in coordination with Institut Pasteur of Bangui and the Ministry of Health in CAR.
The company collaborates with Turnstone Biologics to use TPOXX® as a rescue therapy for vaccinia complications in Turnstone’s oncolytic immunotherapy clinical development program. Siga is also collaborating with Bioarchitech to evaluate the efficacy of TPOXX® in combination with Bioarchitech’s proprietary vaccinia-based immunotherapy platform, which will test the prospects of TPOXX for delivering large doses of oncolytic therapy.
Weakness
In September 2014, the Company filed for voluntary bankruptcy protection to be able to supply its antiviral smallpox drug to the U.S. Strategic National Stockpile. The bankruptcy filing was to safeguard its right to appeal a costly lawsuit over its smallpox drug, Tecovirimat. The Company was found liable to PharmAthene in a licensing dispute, which would result in a damages award of nearly $232 million.
Siga was ordered to pay PharmAthene Inc $113.1 million in damages by the Delaware Court of Chancery for failing to execute a licensing agreement. The Company had to pay PharmaAthene approximately $205 million-plus interest and including pre-judgment interest and varying percentages of PharmAthene’s reasonable attorneys’ and expert witness fees.
In April 2016, the Company announced that it had successfully emerged from Chapter 11.
Opportunity
Smallpox is a contagious disease caused by the variola virus, which caused millions of deaths before being completely eradicated by 1980 by a global immunization campaign. The disease can be transmitted directly from person to person or contracted from an infected person or contaminated items. The infected person starts exhibiting symptoms of the disease 10 – 14 days after being infected, with a typical incubation period of 7 to 17 days, where they cannot transmit the disease.
The incubation period is followed by flu-like symptoms such as fever, headache, fatigue, back pain, and discomfort. This is followed by flat red spots on various parts of the body like the face, hands, forearms, and trunk. These advance into blisters filled with clear water and later pus and turn into scabs after 8 or 9 days later, which leave deep scars when they fall off.
There is no treatment available for smallpox. However, two vaccines exist that can prevent or lessen the severity of the disease. While ACAM2000 uses a live virus, it can cause severe complications and is not recommended for everyone. Jynneos, a modified Ankara vaccine, is deemed safe for even those who cannot take ACAM2000 or have a weakened immunity system. According to a report by brand essence research, the global smallpox vaccine market is poised to grow by a 3.1% CAGR from $63.46 million in 2020 to $78.58 million in 2027.
Given the growing cases of Monkeypox around the globe, there is increased focus on vaccinations that can prevent the disease from spreading and becoming the next epidemic. There is no treatment for Monkeypox at present, but efforts are on to control the outbreak. The CDC guideline recommends the Smallpox vaccine, cidofovir, ST-246, and vaccinia immune globulin (VIG) to control the outbreak of Monkeypox. JYNNEOS, also known as Imvamune or Imvanex, is currently licensed to be used as a preventive measure against monkeypox and smallpox. Information from a clinical study on the immunogenicity of JYNNEOs and animal studies in Africa suggest that JYNNEOS is 85% effective in preventing monkeypox.
Threat
Clinical Trials are fraught with risk and uncertainty. However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to muster a regulatory approval. The success of its clinical trials will allow the Company to advance its pipeline, but it should also be prepared to face any setbacks if its ongoing trials fail to meet their endpoints.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.mayoclinic.org/diseases-conditions/smallpox/symptoms-causes/syc-20353027
https://www.reuters.com/article/siga-tech-bankruptcy-idUKL3N0RH40A20140916
https://investor.siga.com/static-files/958e7ba9-46eb-4139-917d-6d78db993e9f





No Comments