
09 Aug 4 Biotech Stocks for Your Investment Watchlist!
Biotech Companies continue to show resurgence with more and more companies persevering with bringing their clinical candidates to fruition. While Vaccine development and therapeutics to combat the COVID-19 pandemic are at the forefront of development activities in the biotech sector, there are some companies that are making strides in other related fields such as immune-oncology, hematologic disorders and rare immune diseases. Some of these companies have entered into strategic collaborations with some major pharmaceutical companies and have upcoming catalysts in the form of data readouts, initiation of clinical trials and IND submission to look forward to. We take a look at some of them below:
F-star Therapeutics, Inc. (NASDAQ: FSTX)
Market Cap: $113M; Current Share Price: 5.84 USD
Data by YCharts
The Company is developing a pipeline of next-generation bispecifics for cancer immunotherapy that harnesses the power of the immune system to prevent tumors to treat cancer. The Company’s proprietary bispecific antibody platform enables the creation of tetravalent mAb2 bispecifics that can bind to two different antigens at the same time. These antibodies offer developability advantages and do not require additional domain assembly and other modifications.

Image Source: Company
F-Star’s pipeline consists of FS118, a first-in-class LAG3/PDL1 mAb2 bispecific antibody intended for rescuing CPI treatment failures; FS222, a CD137/PD-L1 mAb² bispecific antibody that can improve outcomes in PD-L1 low tumors; FS120, a First-in-class CD137/OX40 mAb² dual agonist bispecific antibody targeting the improvement of CPI and chemotherapy outcomes and SB11285, a Second-generation STING agonist for intravenous administration with potential to improve CPI outcomes.

Image Source: Company
Furthermore, the Company’s partnered programs include DNL-310, intended for the treatment of Hunter Syndrome and multiple blood brain barrier programs being developed in partnership with Denali Therapeutics Inc.
The Company has built a robust intellectual property rights portfolio with over 230 issued patents and more than 180 pending applications that protect its core technology and product line including both the Fcab and mAb2 technologies and their applications.
F-star is helmed by an experienced management team that has a track record of bringing 20 drugs to the market till date and have a wealth of experience in developing biologics. The Company has built strategic partnerships with industry leading pharmaceutical companies such as Merck, AstraZeneca and Denali Therapeutics. The clinical trial collaboration and supply agreement with Merck is to study the combination of FS120, a first-in-class dual-agonist tetravalent bispecific antibody and KEYTRUDA® (pembrolizumab).
The Company has also entered into an exclusive licensing agreement with AstraZeneca for the global rights to the company’s next-generation Stimulator of Interferon Genes (STING) inhibitor compounds. F-Star will receive upfront and near-term payments of up to $12 million, in addition to development and sales milestone payments of over $300 million- and single-digit percentage royalty payments as per a company statement.
Rigel Pharmaceuticals, Inc (NASDAQ: RIGL)
Market Cap: $650.94M; Current Share Price: 3.81 USD
Data by YCharts
Rigel Pharmaceuticals is focused on developing innovative small molecule drugs that target hematologic disorders, cancer and rare immune diseases. The Company’s first FDA approved product is TAVALISSE®* (fostamatinib disodium hexahydrate), an oral spleen tyrosine kinase (SYK) inhibitor, intended for patients with chronic immune thrombocytopenia (ITP), who have had an insufficient response to a previous treatment. ITP is a rare autoimmune disorder in which the immune system attacks platelets in the blood. The drug has demonstrated increased platelet count within a short period from start of treatment and a lasting treatment benefit of up to 3 years and beyond.
The Company announced positive topline results from a phase 2 evaluation of fostamatinib, an oral spleen tyrosine kinase (SYK) inhibitor, intended for the treatment of hospitalized patients with COVID-19.
In April 2021, the Company entered into a licensing agreement with Eli Lilly to co-develop and commercialize Rigel’s R552, a receptor-interacting serine/threonine-protein kinase 1 (RIPK1) inhibitor, indicated for the treatment of autoimmune and inflammatory diseases. Lilly will lead the clinical development of penetrating RIPK1 inhibitors in central nervous system (CNS) diseases as per a company statement. Rigel has received $125 million as upfront cash payment and is eligible to receive an additional $835 million in potential development, regulatory, and commercial milestone payments. Lilly will be responsible for the global commercialization of R552, while Rigel has the rights to the U.S market.
The Company’s pipeline consists of a phase 3 clinical evaluation of TAVALISSE in warm antibody autoimmune hemolytic anemia (AIHA), a blood disorder in which the immune system creates antibodies that damage haemolysis of the body’s own red blood cells (RBC). The candidate has already been granted an Orphan Drug designation and Fast Track designation by the FDA.
Rigel’s lead candidate for the treatment of inflammatory conditions such as psoriasis, rheumatoid arthritis, lupus, multiple sclerosis, inflammatory bowel disease and gout is an investigational drug candidate named R835. The candidate is a potent and selective inhibitor of IRAK1 and IRAK4 that blocks inflammatory cytokine production in response to toll-like receptor (TLR) and the interleukin-1 family receptor (IL-1R) signalling as per a Company statement.
R835 inhibits the production of cytokine in response to TLR and IL-1R activation in vitro and is being tested in a phase 1 clinical study in healthy subjects. The study aims to prove the safety, tolerability and efficacy of R835 in inhibiting both the IRAK1 and IRAK4 signalling pathways, which play a crucial role in inflammation and immune responses to tissue damage.
In June 2011, the Company entered into a strategic collaboration with BerGenBio for an exclusive worldwide research, development and commercialization agreement for R428 (now referred to as bemcentinib), a AXL receptor tyrosine kinase (AXL) inhibitor, indicated for the treatment of immune-evasive, therapy resistant cancers such as acute myeloid leukemia (AML), non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), and melanoma. The Company has received an upfront payment and is eligible to receive milestone payments, sublicensing revenue as well as tiered royalties.
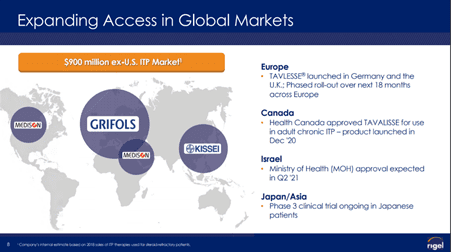
Image Source: Company
Furthermore, Rigel has entered into a collaboration with Daiichi-Sankyo (Daiichi) for development of Rain32 (DS-3032), an oral selective MDM2 inhibitor, indicated for the treatment of haematological malignancies such as relapsed/refractory AML and high-risk MDS. The Company has received an upfront payment and will receive milestone payments and royalties.
The Company is developing R256 (now referred as AZD0449), an inhaled Janus tyrosine kinase (JAK) inhibitor, in association with AstraZeneca, for the treatment of chronic asthma. The candidate is currently being evaluated in a phase 1 clinical trial. Rigel has received an upfront payment and is eligible to receive developmental, regulatory, and launch milestones payments as well as tiered-royalties.
Rigel is also collaborating with Aclaris Therapeutics, Grifols, Kissei and Medison for the developing assets discovered in its laboratory.
Crinetics Pharmaceuticals, Inc. (NASDAQ: CRNX)
Market Cap: $661.64M; Current Share Price: 17.60 USD
Data by YCharts
Crinetics is developing novel therapeutics for the treatment of rare endocrine diseases. The Company’s lead drug candidate Paltusotine is an oral SST2 agonist intended for the treatment of Acromegaly, which is entering the Phase 3 stage. The PATHFNDR phase 3 trial aims to support the potential for broad first-line medical therapy. The Company plans to initiate two double-blind, placebo-controlled studies in the U.S and Europe for both acromegaly patients switching from injectable octreotide or lanreotide depots, who are currently biochemically controlled and untreated acromegaly patients, who are biochemically uncontrolled. These studies are likely to be initiated in 2H,2021. Crinetics expects to report topline data from PATHFNDR – 1 & 2 trials in 2023.
The other programs in the company’s pipeline include a phase 1 trial evaluating Paltusotine for the treatment of Neuroendocrine tumors, CRN04777, an oral SST5 agonist for treatment of Congenital Hyperinsulinism (Congenital HI) and CRN04894, an oral ACTH antagonist targeting the treatment of Cushing’s disease and congenital adrenal hyperplasia (CAH). The Company intends to initiate a phase 2 NET trial in carcinoid syndrome by the end of 2021.
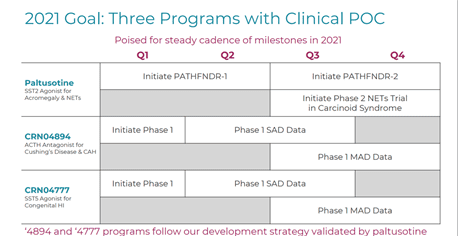
Image Source: Company
The Company expects to report phase 1 SAD data pertaining to CRN04894 in mid-2021 and the MAD data in 2H, 2021. Crinetics has been awarded a Rare Pediatric Disease and EU Orphan Drug Designations for CRN04777, and may be eligible for a priority review voucher in the U.S.
Crinetics has built a strong patent portfolio that covers composition of matter and new formulations that afford it protection until 2037 at the very least.
Kaleido Biosciences, Inc. (NASDAQ: KLDO)
Market Cap: $251.71M; Current Share Price: 5.92 USD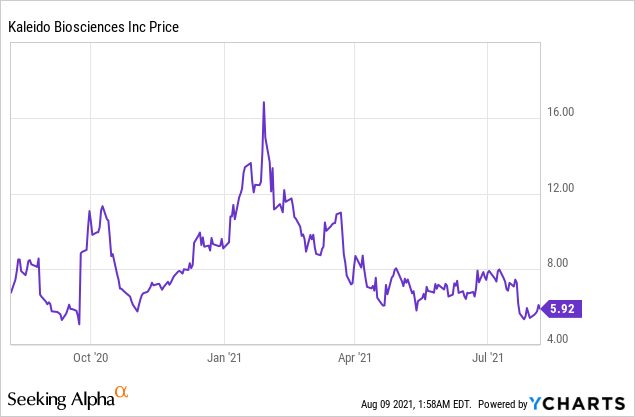
Data by YCharts
The Company is leveraging a proprietary chemistry-driven product platform for development of Microbiome Metabolic Therapies that address disease areas with large unmet patient needs.
Kaleido’s novel approach consists of driving functional outputs of the microbiome through its chemistry-driven approach that also enables the rapid and cost-effective development of innovative product candidates.
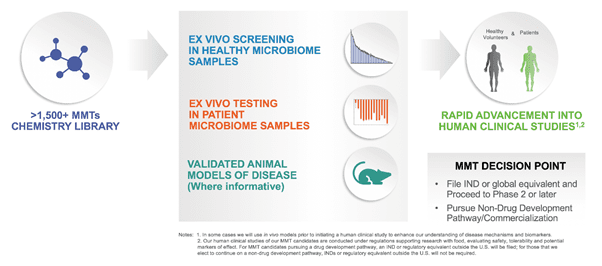
Image Source: Company
Kaleido has a library of over 1500 MMT’s that can target enzymes across taxa, are considered safe, are backed by robust IP and can be orally administered with minimal peripheral exposure. The Company’s pipeline consists of KB109, intended for treatment of patients with mild moderate SARS-COV-2 and patients with high risk for MDR infections and KB295 targeting inflammatory bowel diseases, which is undergoing clinical study in patients with Ulcerative Colitis. Furthermore, the company is developing KB195 for the treatment of Urea Cycle Disorders, which is currently being evaluated in a phase 2 clinical trial and KB174 intended for the treatment of patients with Hepatic Encephalopathy.

Image Source: Company
The Company has entered into strategic collaborations with Janssen for a preclinical program aimed at prevention of childhood-onset Atopic and Immune Conditions and with Gustave Roussy for a preclinical program in immune-oncology. The Company is also pursuing programs in Cardiometabolic & Liver Diseases and Immune-mediated Diseases.
Kaleido conducts clinical studies to support research with food, taking into account safety, tolerability and other potential markers. In addition, the Company engages in clinical trials under IND or similar regulatory equivalent outside the U.S, even for Phase 2 or later development. This enables the company to expedite the discovery and development process and offers improvement over the conventional development approach.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.rigel.com/pipeline
https://investors.f-star.com/static-files/46d708e4-ec22-4e75-b4f5-cedbc52a85bc
https://ir.crinetics.com/static-files/38357d35-7a3e-4b99-82fc-8879dde98631





No Comments