
13 Dec 4 Promising Biotech Stocks with Immense Potential!
Clinical-Stage companies offer an exciting investment opportunity with massive upside potential. Most of these companies bring new and highly differentiated approaches, advanced scientific knowledge and a zeal for innovation to the table.
Our biotech picks that are making strides in fields such as Hepatitis B, rare diseases and dermatology. Some of these companies have entered into strategic collaborations with some major pharmaceutical companies and have upcoming catalysts in the form of data readouts, initiation of clinical trials and IND submission to look forward to.
However, a word of caution is in order as clinical trials are fraught with risk and uncertainty. Even the slightest setback can prove detrimental to the existence of these companies. Failure to meet clinical endpoints, lack of funding or rejection from regulatory authorities are risks that these companies have to bear in pursuit of excellence.
Catalyst Pharmaceuticals, Inc (NASDAQ: CPRX)
Market Cap: $679.55M; Current Share Price: 6.59 USD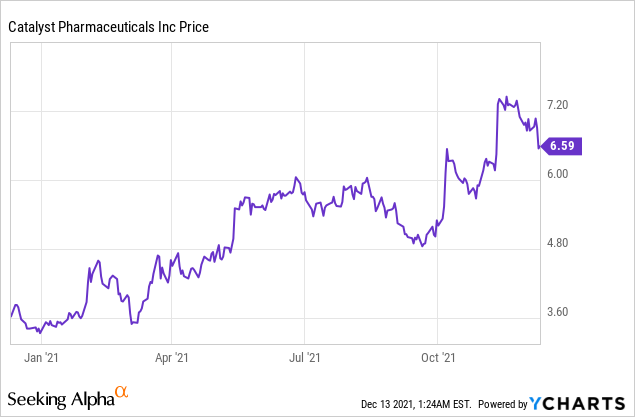
Data by YCharts
Catalyst Pharmaceuticals announced the initiation of a Phase 3 registrational study by DyDo Pharma, the Company’s collaboration partner in Japan, for FIRDAPSE® (amifampridine) 10 mg tablets intended for the treatment of Lambert-Eaton myasthenic syndrome. In June, 2021, the Company entered into a sub-license agreement with DyDo for development and commercialization of FIRDAPSE in Japan, with Catalyst providing clinical and technical support to DyDo so that it can obtain a regulatory approval in Japan. The Company is eligible to receive development and sales-based milestone payment, besides revenue for clinical and commercial supply of the product.
The Company’s lead candidate is an oral nonspecific, voltage-dependent, potassium (K+) channel blocker that has already been approved for treatment of adults with LEMS in the U.S, Europe and Canada. To its credit, amifampridine, is the first FDA-approved product for the treatment of adult patients with Lambert-Eaton Myasthenic Syndrome (LEMS), and the Company’s first product to gain regulatory approval. The approval was based on data from two Phase 3 trials.

Image Source: Company
Catalyst is now focused on expanding its pipeline and is currently engaged in a Phase 3 trial studying the efficacy of amifampridine in patients with MuSK-positive myasthenia gravis (MuSK-MG). Additionally, the Company is preparing to explore the potential of amifampridine in treating other ultra-rare neuromuscular conditions such as congenital myasthenic syndromes (CMS), MuSK-positive myasthenia gravis (MuSK-MG), and spinal muscular atrophy (SMA), besides working on a GABA-aminotransferase (GABA-AT) inhibitor for the treatment of infantile spasms.
The Company reported a record quarter for net sales for FIRDAPSE® and is currently working on expanding the label to include all LEMS patients, including pediatric patients. The revenue from FIRDAPSE stood at $35.9 million in Q3,2021, a 23.1% increase compared to $29.2 million for the third quarter of 2020. The Company also announced its decision to end the development program of Musk-MG as per its Q3,2021 financial results.
Catalyst also received a favorable decision from the U.S. 11th Circuit Court of Appeals supporting orphan drug exclusivity for FIRDAPSE in view of FDA’s approval of, Ruzurgi, another amifampridine product targeting pediatric patients with Lambert-Eaton myasthenic syndrome (LEMS).
Krystal Biotech, Inc. (NASDAQ: KRYS)
Market Cap: $1.78B; Current Share Price: 71.55 USD
Data by YCharts
Krystal is leveraging its proprietary Skin TARgeted Delivery (STAR-D) platform to create novel topical and intradermal “off-the-shelf” therapies for dermatological indications with large unmet needs. The Company is developing a viral gene therapy platform by using the properties of type 1 herpes simplex virus (HSV-1), and creating a non-invasive therapeutic option for treating skin conditions.
The Company’s initial focus is on addressing orphan and rare diseases including monogenic and congenital skin diseases such as epidermolysis bullosa (DEB) and autosomal recessive congenital ichthyosis (ARCI). Krystal’s lead product candidate is Beremagene geperpavec (B-VEC), which has the potential to deliver wild-type human type VII collagen genes for molecular correction of a patient’s fragile skin in dystrophic epidermolysis bullosa (DEB). The candidate is currently being evaluated in a phase III clinical trial.
In November, 2021, the Company announced positive data from a Phase 3 trial named GEM-3, which met its primary endpoint and second endpoint of wound healing at six-month and three-month timepoints. The drug was also well-tolerated and did not have any drug related adverse events or discontinuations. The Company now intends to file a biologics license application (BLA) to the FDA in the first half of 2022 and follow it up with a submission for a marketing authorization application in Europe.

Image Source: Company
Krystal is also developing KB105 for the treatment of autosomal recessive congenital ichthyosis (ARCI), which is a part of an ongoing phase I/II clinical trial. The Company has built a 4,500 square foot state-of-the-art Good Manufacturing Practice (GMP) facility, near its headquarters in Pittsburgh, for meeting the manufacturing requirements of B-VEC. In addition, the Company is also building a commercial gene therapy facility, named ASTRA, that seeks to integrate all components of the supply chain and act as a commercial back-up facility for B-VEC.
The Company’s pipeline also consists of KB-301, a type III collagen that is currently being evaluated in a Phase 1 / 2 and the results from a Phase 1 efficacy are expected to be announced in the second half of 2021. Krystal also has multiple candidates under preclinical evaluation that target chronic skin conditions, Cystic Fibrosis, Netherton Syndrome and other Aesthetic Skin Conditions.
Most recently, the Company received an approval from the Human Research Ethics Committee in Australia for initiating a phase 1 trial of KB407 in Cystic Fibrosis.
Xenon Pharmaceuticals (NASDAQ: XENE)
Market Cap: $ 1.33B; Current Share Price: 25.85 USD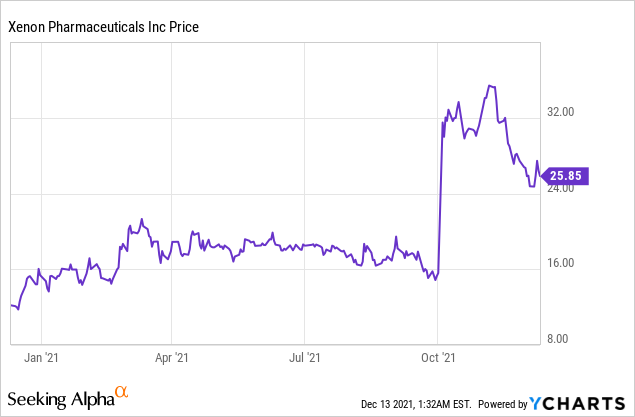
Data by YCharts
A clinical stage biopharmaceutical company, Xenon is developing a neurology-focused pipeline that uses a precision medicine approach to treat chronic neurological conditions including epilepsy. The company’s forte lies in developing highly-selective small-molecule ion channel inhibitors that can target ion channels selectively. Xenon’s area of focus is human channelopathies where it seeks to build on its competence of identifying new binding sites on ion channels leading to discovery of highly selective voltage-gated sodium channel inhibitors with improved safety profiles. It has collaborated with Genentech for the aforementioned purpose.

Image Source: Company
In October 2021, the Company announced positive topline data from the Phase 2b X-TOLE clinical trial which demonstrated statistically significant dose-dependent reduction from baseline in monthly focal seizure frequency when compared to placebo (monotonic dose response; p<0.001), which was the primary endpoint. The median percent reduction was 52.8% (2-sided p-value <0.001) in the XEN1101 25 mg group, 46.4% (2-sided p-value <0.001) in the XEN1101 20 mg group and 33.2% (2-sided p-value = 0.035) in the XEN1101 10 mg group compared to 18.2% in the placebo group as per the Company. The candidate has also shown clear and statistically significant dose response with consistency and improvements in CGI-C and PGI-C for the 25 mg group. XEN1101 was well-tolerated and adverse events reported were dizziness (n=52, 24.6%), somnolence (n=33, 15.6%), fatigue (n=23, 10.9%), and headache (n=21, 10.0%), in line with commonly used ASM’s.

Image Source: Company
Xenon has been awarded an orphan drug designation (ODD) from the FDA for XEN496 as a treatment of KCNQ2-EE otherwise known as EIEE7, a chronic and rare neurodevelopmental disorder which results in seizures and developmental impairment. It also has an ODD from the FDA for XEN007 for the treatment of HM hemiplegic migraine (HM) and alternating hemiplegia of childhood (AHC).
The Company’s pipeline consists of XEN496 (Potassium Channel Opener) currently undergoing Phase 3 evaluation in Orphan Pediatric Epilepsy; XEN1101 (Potassium Channel Opener) in adult focal epilepsy (Phase 2), XEN1101* (Potassium Channel Opener) in Major Depressive Disorder (MDD) being developed in collaboration with Mount Sinai (Phase 2) and another trail in Major Depressive Disorder (Phase 1).
Xenon is also developing NBI-921352 (XEN901) intended for the treatment of Rare Pediatric Epilepsy in collaboration with Neurocrine Biosciences, along with NBI-921352 (XEN901) for treatment of Focal-Onset Seizures in Adults. The Company received $10M in regulatory milestones in September 2021 and can receive a potential $15M on achieving the next regulatory milestone. In addition, the Company is also developing FX301 (Topical NaV1.7 Inhibitor) for Post-operative Pain in partnership with Flexion therapeutics and expects to announce the topline results from a 1 Phase 1b POC clinical trial in Q1,2022.
VBI Vaccines Inc. (NASDAQ: VBIV)
Market Cap: $ 622.68M; Current Share Price: 2.42 USD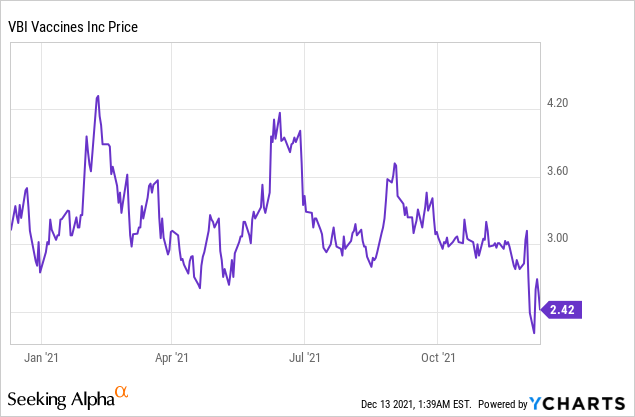
Data by YCharts
In December 2021, VBI Vaccines gained an approval from the U.S. Food and Drug Administration (FDA) for PreHevbrio™ [Hepatitis B Vaccine (Recombinant)] that is intended for the prevention of infection caused by all known subtypes of hepatitis B virus (HBV) in adults age 18 years and older. The vaccine is the only approved 3-antigen HBV vaccine for adults in the U.S. containing the S, pre-S2, and pre-S1 HBV surface antigens.

Image Source: Company
The approval was based on the results from two Phase 3 clinical studies, PROTECT and CONSTANT, which compared PreHevbrio to Engerix-B, a single-antigen HBV vaccine. Data shows that the candidate had higher rates of seroprotection in all subjects age 18+ (91.4% vs. 76.5%), including in adults age 45+ (89.4% vs. 73.1%), with the vaccine being well-tolerated with no unexpected reactogenicity. The vaccine will be made available in the U.S in Q1,2022. The Company is working on getting approvals from the European Medicines Agency’s (EMA) and United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) in 2022. Most recently, the Company announced the filing of a New Drug Submission (NDS) to Health Canada for its 3-antigen prophylactic hepatitis B vaccine candidate.
The Company has an extensive pipeline of prophylactic and therapeutic candidates. VBI has two Phase 1 programs in Cytomegalovirus (VBI-1501) and COVID-19 (VBI-2902) along with preclinical programs in COVID-19 (B.1.351 Variant), Coronavirus and Zika Virus as part of its Prophylactic pipeline. The Company is also evaluating VBI-2601 (BRII-179) in a phase 2 clinical trial and has another Phase 2 program in Glioblastoma (GBM).
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.krystalbio.com/focus/pipeline/
https://investor.xenon-pharma.com/static-files/152c154a-97aa-4ff3-bf90-21e95e1448d8
https://investor.xenon-pharma.com/static-files/152c154a-97aa-4ff3-bf90-21e95e1448d8
https://www.vbivaccines.com/press-releases/prehevbrio-approval/
https://www.vbivaccines.com/wp-content/uploads/2021/10/VBIV-Corporate-Overview-Oct-2021.pdf





No Comments