
27 Oct Should BeyondSpring be a part of your Portfolio?
BeyondSpring, Inc. (NASDAQ: BYSI) is a clinical-stage biopharmaceutical company focused on creating transformative treatments for immune-oncology conditions. The Company’s lead candidate is plinabulin, a selective immunomodulating microtubule-binding agent (SIMBA), which possess a unique mechanism of action and was evaluated in Phase 3 clinical trials in chemotherapy-induced neutropenia (CIN) as well as late-stage non-small cell lung cancer (NSCLC) and other solid tumors.
BeyondSpring, Inc. (NASDAQ: BYSI)
Market Cap: $594.94M; Current Share Price: 15.21 USD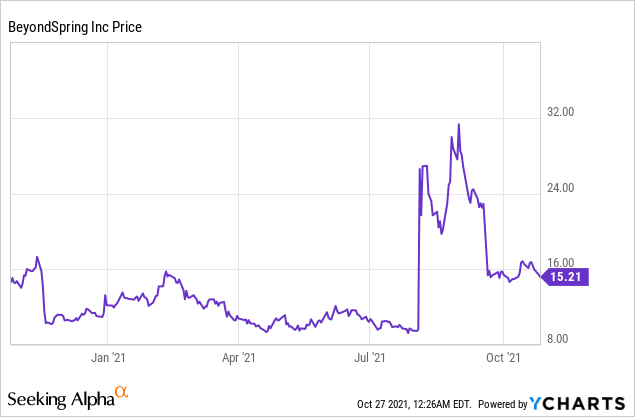
Data by YCharts
Strength
The Company’s lead asset, Plinabulin, is currently undergoing clinical evaluation in two global multi-center trials namely PROTECTIVE-1 (Study 105) and PROTECTIVE-2 (Study 106). In PROTECTIVE-1 (Study 105), a phase 2/3 trial, the candidate is being evaluated in combination with Neulasta for the treatment of advanced breast cancer, hormone-refractory prostate cancer, and advanced NSCLC patients. As part of the PROTECTIVE-2 (Study 106), a phase 2/3 trial, Plinabulin is being tested in combination with Neulasta versus plinabulin monotherapy versus Neulasta monotherapy after a patient has undergone a chemotherapeutic regimen consisting of Taxotere (docetaxel), Adriamycin (doxorubicin) and Cytoxan (cyclophosphamide) in patients with advanced breast cancer.
Plinabulin has demonstrated anticancer properties and the potential to improve clinical outcomes in neutropenia, prevent immune suppression, and reduce bone pain in patients being treated by high-risk chemotherapy. Neutropenia can cause serious patient adherence issues, but treatment with plinabulin/G-CSF combination helps patients adhere to their target doses and cycle times better, thereby improving long-term outcomes.

Image Source: Company
Furthermore, the candidate is also being evaluated in a phase 3 global, multi-centre clinical trial DUBLIN-3 (Study 103), in combination with docetaxel for the treatment of advanced NSCLC, in the U.S., China, and Australia. Plinabulin has shown the ability to work at the early stages in the process of immune activation against cancer, specifically activating dendritic cells and mobilizing tumor antigen-specific T cells to the tumor. The candidate has also been found to act synergistically with checkpoint inhibitors, improving their antitumor efficacy. The Company believes that plinabulin in combination with nivolumab, will be able to enhance antitumor activity, with minimal toxicity, which has proven to be a challenge so far when nivolumab is combined with other checkpoint inhibitors.
In addition, the Company is also engaged in a phase 1 clinical trial, with a triple combination of plinabulin, nivolumab, and ipilimumab, for the treatment of small cell lung cancer, in collaboration with the Big Ten Cancer Research Consortium. BYSI has also entered into a sponsored research agreement with MD Anderson to evaluate the benefit of adding plinabulin to radiation therapy plus immune checkpoint antibodies, which demonstrated that a triple combination approach (plinabulin + radiation + PD-1 antibody) is highly effective in tumor reduction, increasing tumor dendritic cell maturation and increasing tumor T-cell infiltration.
The Company has already submitted a New Drug Application (NDA) for plinabulin in CIN prevention to the U.S FDA and in China in March 2021 and has been granted an FDA priority review with a PDUFA date of November 30, 2021. The candidate has been granted a breakthrough designation and a priority review from both the entities.
In August 2021, the Company reported positive top-line data of DUBLIN-3 registrational trial in plinabulin in combination with docetaxel to treat 2nd and 3rd line non-small cell lung cancer. The data shows that the combination met the primary endpoint of overall survival and secondary endpoints of significant improvement in ORR, PFS and 24- and 36-month OS rates, and significant reduction in the incidence of Grade 4 neutropenia. The Company intends to submit an NDA in the first half of 2022.
The Company owns the global rights to plinabulin, except for China. In China, the company owns a 57.97% stake in its Chinese subsidiary, Dalian Wanchunbulin Pharmaceuticals Ltd, which owns full rights to plinabulin. BYSI has entered a co-development and commercial partnership with Hengrui in Greater China. The Company also has subsidiary SEED therapeutics, which signed an $800 million research collaboration with Eli Lilly based on its “molecular glue” TPD platform.
BYSI’s pipeline also consists of BPI-002, an oral small molecule agent that increases T-cell co-stimulation; BPI-003, a novel small molecule inhibitor of IKK, a protein kinase and BPI-004, a small molecule that induces the tumor cells to produce antigens.
The Company has built strategic partnerships and collaborations with the University of Washington, NYU, UC San Diego, Fred Hutch, John Hopkins University, Rutgers, MD Anderson Cancer Centre among others.
Weakness
Plinabulin belongs to the class of vascular disrupting agents that have infamously failed in multiple clinical tests over the years. Some of the most noteworthy vascular disrupting agents currently under evaluation are BNC105 from Bionomics and MN-029/ denibulin from Medicinova, that are currently being evaluated in a Phase Ph1 (US) & ph2 (Australia) investigator-initiated trial in Australia and two Phase 1 trials respectively.
However, there is a long list of VDA that have failed to achieve their objectives in clinical trials previously such as Vadimezan from Antisoma/Novartis that failed in a Phase 3 trial in NSCLC; Ombrabulin from Sanofi that could not achieve its objectives in a Phase 3 trial in soft tissue sarcoma and ZD6126, that was scrapped after Phase 2 trials by AstraZeneca and later by Angiogene to name a few.
The Company’s NDA for Plinabulin in chemotherapy-induced neutropenia is based on Phase 3 trial data with subjects enrolled in China and Ukraine, with no US patients participating in the Phase 3 registrational trial. This could prove to be detrimental to the prospects of its NDA being approved.
In August 2021, the Company reported positive topline results from its DUBLIN-3 registrational trial of Plinabulin in combination with docetaxel to treat 2nd and 3rd line NSCLC (EGFR wild type), however, its primary overall survival endpoint value was stated to be <0.04 p, which might not be very significant. In addition, the median OS was not disclosed nor were any actual values including omission of Hazard Ratio (HR). Meanwhile, an SEC filing shows the HR to be 0.82, which is barely significant.
Most importantly the data fails to reflect the current treatment practices in NSCLC that use a PD-(L)1 inhibitor as a front-line treatment. During an analyst call, the Company stated that only 15 percent of the 559 patients enrolled in Dublin-3, a second/third-line NSCLC trial had failed PD-(L)1 blockade. The trial is intended to support an NDA filing in the first half of 2022. More information about how the drug performs after treatment with Keytruda as opposed to evaluating a post-chemo effect in patients who switched from platinum to docetaxel chemo would help strengthen its case.
One reason for this could be because the trial was initiated in 2015, way before any anti-PD-(L)1 therapy was even approved by the FDA as a front-line treatment for NSCLC. The candidate was originally developed by Nereus Pharmaceuticals, which was acquired by Triphase Accelerator in 2012. BYSI bought plinabulin from Triphase Accelerator and is awaiting a nod in chemotherapy-induced neutropenia, with a November 30 PDUFA date.
Another moot point is that the US patient population constitutes only 20 percent of the trial as per CEO Lan Huang, implying that the rest 80 percent were from Australia and China, which may not fully represent a typical US population. Race and ethnicity play an important role in how drugs react and will play a crucial role in the FDA’s decision. However, the Company states that it has received reassurance that if the pharmacokinetic data of the eastern patients is like those of the US, the FDA would not have any objection to the US filing. The trial data consists of patients who were undergoing TAC chemotherapy, which isn’t used very frequently in the U.S. The company has repeatedly changed primary and secondary endpoints of its trials, which raise concerns about inconsistency, claims of effectiveness and lack of transparency.
Opportunity
Neutropenia is characterized by low neutrophil, which are a type of white blood cells produced by the bone marrow that help the body fight infections by eliminating harmful viruses and bacteria in the body. Treatment with chemotherapy causes neutropenia and puts patients at a greater risk of developing infections. The disease manifests in the form of high fever, chills, abdominal pain, and diarrhoea to name a few. Other causes for Neutropenia include leukemia, lymphoma and radiation therapy. The present options for managing neutropenia include delaying chemotherapy, use of antibiotics, or treatment with white blood cell growth factors.
According to a report by Research and Markets, there were 691,906 patients on Chemotherapy in the U.S in 2020, while the total patients on chemotherapy in the 7MM stood at 1,790,618. Out of these, 484,334 cases at intermediate-high risk for CIN, and 207,572 cases at low risk for CIN in the United States. The market for CIN stood at 3.567 billion in 2020, with Neulasta, Neupogen, and their biosimilars cornering a majority share of the market.
Non-Small Cell Lung Cancer is the most common form of Lung Cancer with an incidence rate of over 85% that manifests as Adenocarcinoma, Large Cell Carcinoma and Squamous Carcinoma. Small cell lung cancer also known as Oat Cell Cancer, which spreads rapidly makes up for around 10% to 15% of the cases and finally Lung carcinoid or neuroendocrine tumors that are less than 5% of the diagnosis and grow slowly and rarely spread.
According to an estimate by the American Cancer Society, there will be over 235,760 new cases of lung cancer (119,100 in men and 116,660 in women) diagnosed in 2021 with about 131,880 deaths resulting from it. The death rate for Lung cancer alone is higher than that of colon, breast, and prostate cancers combined.

Image Source: Freepik
The Global Lung Cancer market is estimated to grow at a CAGR of 13.2%, during 2018-2022, to reach 36.93 billion by 2022 according to a report by Market Research Future. The rise in geriatric population, pollution levels, negative lifestyle conditions such as Active and passive smoking, increased healthcare expenditure and rapid scientific and technological advancements will drive the growth in the market.
The emergence of biomarkers such as epidermal growth factor receptor (EGFR), Anaplastic lymphoma kinase (ALK) and K-ras mutations (KRAS 1) have made the identification and management of lung cancer more accurate and manageable. These also help determine the optimal treatment plan for a patient, thereby avoiding the burden of undergoing treatments that may not be appropriate and the related side-effects.
Threats
Clinical Trials are fraught with risk and uncertainty. There is a possibility that the candidates in the Company’s developmental pipeline may not be able to meet their clinical endpoints in trials. However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to meet endpoints in any of its ongoing trials. The success of its clinical trials will help the Company advance its pipeline, but it should also be prepared to face any setbacks in case its ongoing trials fail to meet their endpoints.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://beyondspringpharma.com/pipeline/plinabulin/
https://beyondspringpharma.com/pipeline/
https://beyondspringpharma.com/wp-content/uploads/2021/10/BYSI-Corporate-Deck-Oct-2021-FINAL-1.pdf
https://www.evaluate.com/vantage/articles/news/trial-results/plinabulin-blooms-last-beyondspring





No Comments