
16 Jun Geron Corporation: A lot is riding on Imetelstat!
Geron Corporation, Inc. (NASDAQ: GERN) is a late-stage clinical biotechnology company developing a first-in-class telomerase inhibitor, imetelstat, intended to treat hematologic malignancies. The Company’s lead candidate Imetelstat is a novel first-in-class telomerase inhibitor currently undergoing a Phase 3 trial, IMerge, which is a double-blind, randomized, placebo-controlled trial with registrational intent. The trial has approximately 170 transfusion-dependent patients with Low or Intermediate-1 risk myelodysplastic syndromes (MDS) or low-risk MDS who have relapsed after or are refractory to prior treatment with an erythropoiesis-stimulating agent (ESA).
The Company is also engaged in a Phase 3 trial named IMpactMF, an open-label, randomized, controlled trial that will enroll approximately 320 patients with Intermediate-2 or High-risk myelofibrosis (MF) who are refractory to prior treatment with a JAK inhibitor.
Imetelstat has been granted a Fast Track designation by the United States Food and Drug Administration (FDA) for transfusion-dependent anemia due to a lower risk of MDS in patients with non-del(5q) and who are refractory or resistant to treatment with an erythropoiesis-stimulating agent, or ESA, and for the treatment of patients with relapsed/refractory MF. The candidate has also been granted an orphan drug designation by the FDA and the European Commission for the European Medicines Agency for treating MDS and MF. The United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) granted imetelstat, an Innovation Passport designation, for reducing the time to market for innovative medicines.
Geron Corporation, Inc. (NASDAQ: GERN)
Market Cap: $490.68M; Current Share Price: 1.30 USD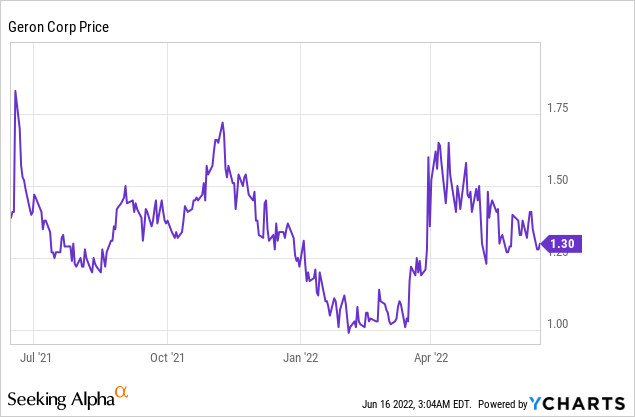
Data by YCharts
We take a holistic look at the Company through a SWOT analysis below:
Strength
The Company’s lead candidate, Imetelstat sodium (imetelstat), formerly known as GRN163L, is a small oligonucleotide composed of nucleic acid and a lipid moiety. The candidate is resistant to degradation, thereby providing improved stability in plasma and tissues and binding affinity to selected targets. In addition, imetelstat offers cell permeability, increased potency, and excellent pharmacokinetic and pharmacodynamic properties. The compound is administered intravenously and has a long residue time in the bone marrow, spleen, and liver. It binds with high affinity to the RNA component of telomerase, leading to direct inhibition of telomerase enzymatic activity.
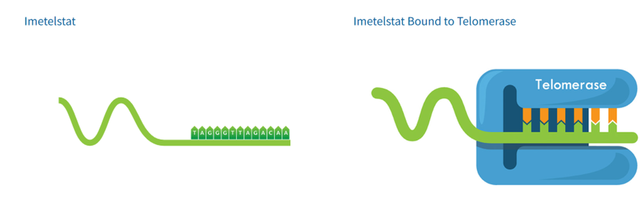
Image Source: Company
In preclinical efficacy studies, the candidate demonstrated the ability to inhibit telomerase activity and shorten telomeres, inhibiting the proliferation of various tumor types and inhibiting the proliferation of malignant progenitor cells such as multiple myeloma myeloproliferative neoplasms and acute myelogenous leukemia.
The candidate has completed two Phase 2 clinical trials in lower-risk MDS and relapsed/refractory myelofibrosis and demonstrated meaningful and durable Transfusion Independence in High Transfusion–Burden Patients with Lower-Risk Myelodysplastic Syndromes. Imetelstat is currently undergoing evaluation in a Phase 2/3 trial in lower-risk myelodysplastic syndromes (MDS), and IMpactMF, a Phase 3 trial in refractory myelofibrosis (MF).
The topline results from the IMerge Phase 3 are expected to be announced early January 2023. Based on the outcome of the trials, the Company intends to submit a New Drug Application (NDA) with the FDA and a Marketing Authorization Application (MAA) with the European Medicines Agency (EMA) in the first and second half of 2023, respectively. The Company is expecting a potential commercial launch of imetelstat in lower-risk MDS in the U.S in the first half of 2024 and Europe in the second half of 2024.
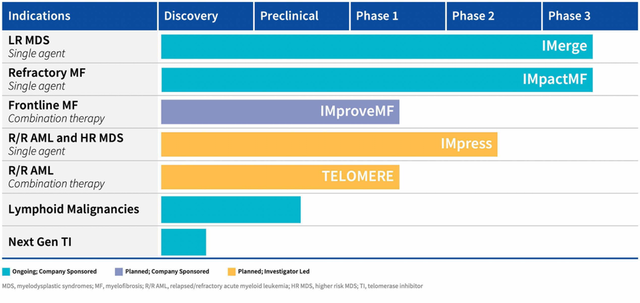
Image Source: Company
Geron’s pipeline consists of additional indications in which imetelstat is being evaluated, such as Frontline MF as a combination therapy currently in Phase 1, as a single agent in R/R AML and HR MDS, and as a combination therapy in R/R AML. The Company is also conducting preclinical studies in Lymphoid malignancies and the Next Generation telomerase Inhibitor Discovery Program.
Weakness
The Company had been collaborating with Johnson and Johnson to develop a cancer drug since 2014. However, in September 2018, Johnson and Johnson biotech unit Jannsen pulled out of the collaboration citing “strategic portfolio evaluation and prioritization of assets.” The decision meant the Geron had to shoulder the responsibility of financing the Phase 2 clinical trials, besides all other funding requirements to advance the candidate to commercial launch.
Geron has had to resort to dilutive share offerings, the latest being a public offering completed in April 2022, to fund regulatory filings for imetelstat.
The Company was founded in 1990 and is yet to receive approval from the Food and Drug Administration (FDA) for any of its candidates. A lot rides on the results from the Phase 3 trial of imetelstat, as it could be a make or break for the company’s fortunes.
Opportunity
Myelodysplastic syndromes are the name given to a group of disorders that disrupt the production of blood cells. Unlike the process in a healthy individual in which the bone marrow makes immature blood cells, also known as “blasts,” which turn into mature cells over time. However, in the case of Myelodysplastic syndromes, the immature cells do not mature or become healthy blood cells or platelets in the bone marrow and end up either dying in the bone marrow or entering the blood, leaving very little space for healthy white blood cells, red blood cells, and platelets.
The disorder can be caused due to past treatment with chemotherapy or exposure to radiation. The typical symptoms associated with these disorders include fatigue, shortness of breath, easy bruising paleness, and Petechiae. Diagnosis is based on the changes in the blood cells and bone marrow. The different manifestations of the disorder include Refractory anemia, refractory anemia with ring sideroblasts, Refractory anemia with excess blasts, Refractory cytopenia with multilineage dysplasia, Refractory cytopenia with unilineage dysplasia, Unclassifiable myelodysplastic syndrome, Myelodysplastic syndrome associated with an isolated del(5q) chromosome abnormality and Chronic myelomonocytic leukemia.
The standard treatment of the condition includes supportive care, drug therapy, and stem cell transplantation. In some instances, the disorder can progress to acute myeloid leukemia.
According to the Leukemia & Lymphoma Society, there are 20,000 new cases of MDS reported every year in the U.S alone. It is estimated that the condition afflicts 60,000 – 170,000 patients in the US, with a median age of 71-76 years.
Threats
The Company’s pipeline primarily consists of only one candidate being evaluated in multiple indications. The other indications that the candidate is being evaluated are either in the preclinical or Phase 1/2 trial stage. Clinical Trials are fraught with risk and uncertainty. There is a possibility that the candidates in the Company’s developmental pipeline may not be able to meet their clinical endpoints in trials.
The Company may fail to receive regulatory approval for imetelstat, resulting in a setback for the other candidates in the pipeline.
However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to meet endpoints in any of its ongoing trials. The success of its clinical trials will allow the Company to advance its pipeline, but it should also be prepared to face any setbacks in case its ongoing attempts fail to meet its endpoints.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References





No Comments