
09 Dec Is Third Time the Charm for Fennec Pharmaceuticals?
Fennec Pharmaceuticals (NASDAQ: FENC), a specialty pharmaceutical company, received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) for its New Drug Application (NDA) for PEDMARK™ (sodium thiosulfate), which was being developed as an intravenous administration for the prevention of ototoxicity associated with cisplatin chemotherapy in pediatric patients ≥ 1 month to 18 years of age with localized, non-metastatic, solid tumors.
In June 2021, the Company had resubmitted its New Drug Application (NDA) for PEDMARK™ and was granted a Prescription Drug User Fee Act (PDUFA) target action date of November 27, 2021. The candidate was granted a Fast-Track Designation and Breakthrough Therapy Designation by the FDA. Fennec had originally submitted an NDA in February 2020, but was issued a CRL in August 2020 due to deficiencies in manufacturing.
The new CRL also raises issues related to manufacturing deficiencies, which need to be resolved before Pedmark can be approved by the FDA. The Company intends to request for a Type A meeting with the FDA to discuss the way forward.
Fennec Pharmaceuticals (NASDAQ: FENC)
Market Cap: $110.79M; Current Share Price: 4.26 USD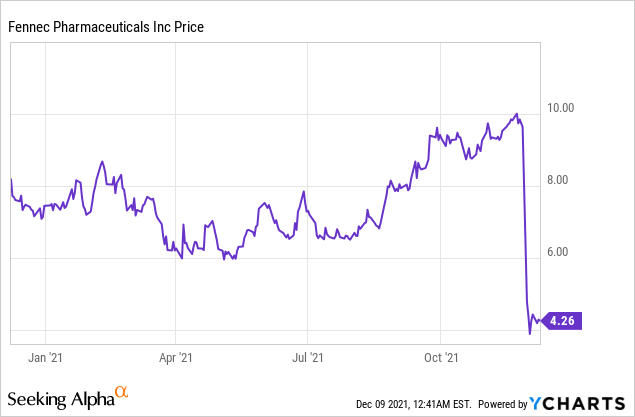
Data by YCharts
Rosty Raykov, chief executive officer of Fennec Pharmaceuticals, commented,
“We are steadfast in our commitment to reducing the risk of life-long hearing loss for children and young adults receiving cisplatin chemotherapy who currently have no approved therapies for this devastating condition. We will work closely with our current manufacturer as well as the FDA to fully address the issues raised in the letter. In addition, we continue to advance our second drug product manufacturing facility.”
PEDMARK has undergone evaluation in two phase 3 clinical studies, namely The Clinical Oncology Group Protocol ACCL0431 and SIOPEL 6. ACCL0431 has evaluated patients with newly diagnosed hepatoblastoma, germ cell tumor, osteosarcoma, neuroblastoma, and medulloblastoma, while SIOPEL 6 enrolled only hepatoblastoma patients with localized tumors as per the Company.
Strength
Fennec’s PEDMARK TM is a novel formulation of Sodium Thiosulfate that is targeted at prevention of Cisplatin Ototoxicity in pediatric patients. Cisplatin is a platinum compound that is part of chemotherapy for pediatric malignancies. Its use invariably leads to ototoxicity and leaves the patient suffering from a lifetime of hearing disabilities. The drug has received an Orphan Drug Designation from the FDA for use in the prevention of platinum-induced ototoxicity in pediatric patients; in addition, it is also eligible for a Pediatric Use Marketing Authorisation (PUMA) status giving it 7 and 10 years of market exclusivity respectively.
Pedmark is a formulation of sodium thiosulfate (STS), a water-soluble thiol compound that can act as a reducing agent. It can inactivate platinum complexes and render them non-cytotoxic by covalently binding electrophilic platinum with thiol; it also provides delayed administration, reduced toxicity and enhanced levels of endogenous reducing agents such as GSH. In two crucial phase III studies the candidate has met the primary efficacy endpoint of hearing loss protection.
Fennec has licensed 1 US and 9 foreign patents from Oregon Health and Science University for STS which expire in 2021 in Europe and Japan. The candidate also enjoys patent protection in the U.S until 2039 for the unique anhydrous form of the active ingredient as well as related methods of synthesis. Furthermore, the Company was granted patent protection until 2038 for methods of use for children <5 years of age.
Weakness
The Company had originally received a CRL in August 2020, due to deficiencies identified at a manufacturing plant used by the Company, and was given a Form 483 listing out the conditions to be met before the candidate could be approved. The Company had over a year to address the deficiencies but has failed to do so. The bright side is that the FDA has not found anything wanting with the clinical trial data at the time of issuing the CRL in August 2020, with CMC issues being the cause for rejection of its NDA.
According to a study evaluating the effects of sodium thiosulfate for protection from cisplatin-induced hearing loss, a post hoc analysis showed survival in the sodium thiosulfate group was significantly lower among those with disseminated disease. Therefore, though sodium thiosulfate is safe to use in patients with standard-risk hepatoblastoma and other localized cancer, it may affect the survival rate in patients with disseminated disease.
This could have an effect on Pedmarks’s total addressable market, as physicians may exercise caution in using the product not only in case of disseminated diseases but also for localized disease as well.
The Company had Cash and cash equivalents of $24.3 million as of September 30, 2021 and $5.0 million in funded debt. The Company will need to raise additional capital to fund its resubmission and commercialization activities, thereby impacting shareholder value.
Even if the drug is approved, the road to commercialization will involve creating patient and physician awareness and assurance that there are no safety concerns, besides considerable investment in building a sales and marketing infrastructure.
Opportunity
Cisplatin, a platinum-based chemotherapy drug is used to treat numerous forms of cancer such as ovarian, cervical, testicular, breast and bladder cancer among others. The drug is considered one of the strongest chemotherapeutic and has been known to cause toxicity. Common side effects of cisplatin include lowered white blood cells, anemia, kidney problems, neuronal damage and irreversible hearing loss.
A study led by researchers from the National Institute on Deafness and other Communication Disorders (NIDCD), part of the National Institutes of Health found that cisplatin is found in the cochlea, months and years after treatment, damaging the cochlea in 40%–80% of adults and nearly 50% of the children and leaving them with permanent hearing loss.
Hearing loss can lead to social isolation, lower quality of life, and higher rates of dementia and depression. Lifetime healthcare costs of untreated profound hearing loss are estimated to cost more than $1 million per person in the U. S alone. The rising incidences of cancer, technological advancements and increased research and development in the field will drive the growth in the market.
Threat
The Company’s pipeline is currently centered around one candidate – Pedmark. While gaining an approval is the final goal, another rejection from the FDA could prove detrimental to the Company’s interest.
Clinical Trials are fraught with risk and uncertainty. However, a diverse pipeline will help mitigate the risk in case of adverse results or the failure to muster a regulatory approval. The success of its clinical trials will help the Company advance its pipeline but it should also be prepared to face any setbacks, in case its ongoing trials fail to meet their endpoints.
Key Takeaways
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://www.cancer.gov/news-events/cancer-currents-blog/2018/cisplatin-hearing-loss





No Comments