
22 Jun 5 Most Promising Alzheimer’s Disease Stock!
Alzheimer’s is the sixth leading cause of death in the U.S, a disease that causes irreversible degeneration of the brain cells and affects a person’s cognitive and behavioral abilities such as impaired judgment, deteriorating memory and personality changes with the gradual loss of motor abilities and speech.
According to some compelling statistics made available by Alzheimer’s Association over 5.8 million Americans are affected by this disorder and the numbers will most likely reach 14 million by 2050, with a new diagnosis being made every 65 seconds. The healthcare cost of Alzheimer’s and other Dementia related ailments will be over $290 billion in 2019, these numbers are likely to escalate further to over $1.1 trillion by 2050.

Image Source: NIH
A report by transparency market research predicts that the global market for Alzheimer’s will reach US$6.4 billion by 2025, growing at a CAGR of 7.5%, from US$3.6 billion in 2017. The rise in ageing population, increased awareness about the benefits of combined therapy, a favorable regulatory and government support environment are some of the factors that will contribute to the growth of the market. The economic burden of the disease on not only the patients but also caregivers will act as an impediment for the market. The prohibitive cost of expensive treatment especially in developing nations is another obstacle for growth.
Alzheimer’s is one illness, where innumerable attempts have been made to find a cure with no success. According to a report released by Pharmaceutical Research and Manufacturers of America (PhRMA) from 1998 to 2017 there have been about 146 failed shots at developing drugs for Alzheimer’s disease. However recent advancements in technology and the discovery of new molecules and combination therapies are offering renewed hope to those suffering from this illness.
In June 2021, Biogen’s ADUHELM™ (aducanumab-avwa), scored an approval from the U.S. Food and Drug Administration (FDA), to become the first drug approved after 2003 for the treatment of Alzheimer’s disease, which specifically addresses the underlying cause of the disease, by removing amyloid beta from the brains of those afflicted with the condition.
We take a look at some of the other most promising candidates in the pipeline to treat Alzheimer Disease:
Annovis Bio, Inc. (NYSE: ANVS)
Market Cap: $667.63M; Current Share Price: 96.10 USD
Data by YCharts
Annovis is a clinical-stage biotechnology Company that is developing a pipeline of drugs that target the treatment of Alzheimer’s and other neurodegenerative diseases through improvement of Axonal Transport. The Company is working on therapeutics for Alzheimer’s disease (AD), Parkinson’s disease (PD), Dementia and Alzheimer’s in Down Syndrome (AD-DS) (granted an orphan drug designation by the FDA).
Annovis’s lead candidate ANVS401, is under clinical development for the treatment of AD-DS, AD and PD and has demonstrated the ability to inhibit neurotoxins and normalize axonal transport in preclinical studies. In addition, the candidate is able to reduce APP/Aβ, tau/phospho-tau and α-synuclein, which are responsible for causing inflammation and cell death. Additionally, ANVS401 was shown to normalize levels of APP, tau and αSYN in the cerebrospinal fluid (CSF), besides being well-tolerated and safe, in its third Phase 1 clinical study.

Image Source: Company
In June 2021, Annovio announced preliminary data from a study initiated last year that has demonstrated the potential of ANVS401 to protect nerve cells from death resulting from an infection by gingipains, the virulence factors of Porphyromonas gingivalis (P. gingivalis). The Company intends to explore the possibility of the candidate being used in the treatment of neurological diseases caused due to bacterial and viral infections.
The Company plans to initially focus on AD-DS in Phase 3 and intends to use the orphan status to obtain human data for AD much faster than in a regular AD population. In studies related to trisomic DS animals and nerve cells, the candidate was able to restore and improve axonal transport, reduce APP, tau and phospho-tau and increased levels of BDNF (a neurotrophic factor), leading to restored memory and learning and returned the brain to homeostasis.
ANVS401 has also been evaluated in mice with Parkinson’s disease and treatment with the candidate has been able to lower aSYN in the gut and in the brain and fully normalizes colonic motility.
The Company’s pipeline also consists of ANVS405, an injectable drug for protecting the brain from TBI and/or stroke in cases of acute brain/head trauma. The development of the candidate is funded by the US Army and the Company intends to apply for further grants for its development. Furthermore, Annovis is also developing ANVS301, intended for the treatment of advanced AD and Dementia, which is currently undergoing a Phase 1 clinical trial being conducted and sponsored by the National Institutes of Health (NIH).
Annovio is currently engaged in a Phase 2a study in AD patients in collaboration with the Alzheimer’s Disease Cooperative Study (ADCS) and a Phase 2a study in AD and PD Patients.
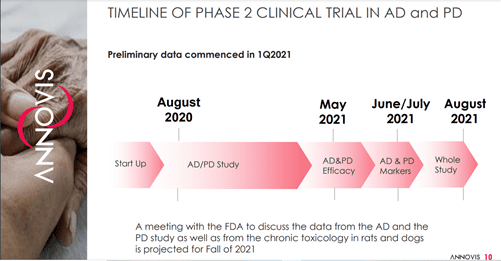
Image Source: Company
The Company has reported data from the AD & PD efficacy trial in May 2021 and will soon release the AD & PD marker data in June/July 2021, with the results from the complete study expected to be announced in August 2021. The results from the Phase 2a trial show statistically significant improvement in parameters such as cognition, motor function, WAIS coding and Inflammation in patients with AD and PD.
Annovis is also planning to meet the FDA in the fall of 2021 to discuss data from the AD and PD study along with data from chronic toxicology in rats and dogs.
Cassava Sciences Inc (NASDAQ: SAVA)
Market Cap: $3.59B; Current Share Price: 89.72 USD
Data by YCharts
Cassava is a clinical-stage company that develops innovative first-in-class medicines for the treatment of neurodegenerative conditions such as Alzheimer’s. The Company’s lead drug candidate is Simufilam, formerly known as PTI-125, which aims to stabilize a critical protein in the brain, by restoring the shape and functioning of a scaffolding protein, namely altered filamin A (FLNA).
The candidate is a proprietary small molecule that has a dual mechanism of action, with the ability to reduce neurodegeneration and neuroinflammation. Simufilam has undergone multiple clinical trials and has demonstrated safety and tolerability in humans. Additionally, it has also shown positive results on CSF biomarkers in an open-label Phase 2a study of Simufilam in AD patients and in a double-blind, randomized, placebo-controlled Phase 2b study of simufilam in AD patients, besides demonstrating positive results on cognition in a 6-month interim analysis of an open-label on-going study.
In addition, the Company is also developing SavaDx, formerly known as PTI-125Dx, a quantitative blood-based diagnostic that can detect Alzheimer’s years before its appearance and has been able to detect more than 10-fold differences between patients with Alzheimer’s and age-matched normal controls in blinded studies. Cassava is collaborating with The National Institutes of Health (NIH) for scientific and financial support for its research programs.
In February 2021, the company announced that it has reached a consensus with the U.S. Food and Drug Administration (FDA), as to key elements of a crucial Phase 3 clinical program for Simufilam, its lead candidate for the treatment of Alzheimer’s. The minutes of the successful End-of-Phase 2 (EOP2) that took place in January 2021, indicate that FDA has agreed to consider the recently completed Phase 2 program, along with its upcoming Phase 3 clinical program, as sufficient to show clinical efficacy for Simufilam in Alzheimer’s disease.
Furthermore, the Phase 3 trial will use separate clinical scales to assess cognition (ADAS-cog1) and function (ADCS-ADL2) as co-primary endpoints and iADRS3, as secondary efficacy endpoints. The first study will enrol nearly 1000 subjects, who will be treated for 18 months and will be initiated in Q3,2021, while the second study will enroll 600 subjects to be treated for 9 to 12 months and is likely to commence in Q4,2021.
The FDA has agreed to review each protocol as well as conduct a Special Protocol Assessment (SPA) for both studies. In addition, the Company also intends to conduct a second interim analysis of the open-label study mid-year 2021 and carry out a Cognition Maintenance Study (CMS) in Q2,2021.
The Company has patent protection for simufilam that includes six issued patents pertaining to composition of matter claims and other novel, filamin-binding molecules, running through 2033. Cassava Sciences holds exclusive worldwide rights to simufilam and has no milestone or royalty obligations to any third party. On the other hand, SavaDx enjoys protection by trade secrets, know-how and proprietary rights technology.
Axsome Therapeutics (NASDAQ: AXSM)
Market Cap: $2.66B; Current Share Price: 70.75 USD
Data by YCharts
Axsome uses its chiral chemistry and formulation capabilities to identify, isolate and stabilize chirally pure enantiomers for developing drug candidates for the treatment of central nervous system (CNS) disorders with large unmet needs. Its proprietary MoSEIC™ technology can increase the solubility and absorption of drug molecules, while its metabolic inhibition technology can increase the bioavailability and prolong the half-life. The company uses its in-depth knowledge of synthesis and analysis for narrowing target drug molecules.
AXS-05, a combination of bupropion and dextromethorphan, is its lead candidate for treatment of agitation associated with Alzheimer’s. Agitation in Alzheimer’s is characterized by aggressive behavior, lack of inhibition and emotional distress, which makes it difficult for caregivers to provide quality care and leads to earlier nursing home placements and increased mortality risk. Over 70 percent of people living with AD suffer from agitation, however there is currently no approved treatment for the solution.
The drug combines Dextromethorphan which is an NMDA receptor antagonist, sigma-1 receptor agonist, and inhibitor of the serotonin and norepinephrine transporters along with Bupropion that increases the bioavailability of dextromethorphan, and is a norepinephrine and dopamine reuptake inhibitor, and a nicotinic acetylcholine receptor antagonist.

Image Source: Company
In April 2020, the Company announced topline results from ADAVANCE-1, a phase 2/3 trial evaluating AXS-05 in Alzheimer’s Disease Agitation. The candidate achieved the primary endpoint of significant improvement in CMAI total score versus placebo and was found to be statistically superior to bupropion. AXS-05 also contributed to improvement in agitation including greater clinical response and 50 percent reduction in agitation symptoms. In addition, AXS-05 was also found to be well-tolerated and safe.

Image Source: Company
In August 2020, the Company had a Breakthrough Therapy meeting with the FDA, which led to the initiation of ACCORD (Assessing Clinical Outcomes in Alzheimer’s Disease Agitation) in December 2020, a Phase 3 trial to evaluate the efficacy and safety of AXS-05 in the treatment of Alzheimer’s disease (AD) agitation. Topline results from the trail are expected to be announced in H2, 2022.
In April 2021, the FDA accepted the filing of the Company’s New Drug Application (NDA) for AXS-05 for the treatment of major depressive disorder (MDD). The candidate has been granted a priority review and given a Prescription Drug User Fee Act (PDUFA) target action date of August 22, 2021.
Furthermore, the Company announced its plan to submit a New Drug Application (NDA) for AXS-14, intended for the treatment of fibromyalgia. The submission is expected to be made in Q4,2022 as the company is currently working on completing the manufacturing and other related activities. AXS-14 (esreboxetine) is a highly selective and potent norepinephrine reuptake inhibitor, which is more potent and selective than racemic reboxetine.
Cortexyme Inc (NASDAQ: CRTX)
Market Cap: $1.66B; Current Share Price: 56.08 USD
Data by YCharts
Cortexyme, a clinical stage pharmaceutical company, is adopting a path breaking, novel disease-modifying therapeutic approach for treating the underlying cause of Alzheimer’s disease and other degenerative diseases. Its lead candidate Atuzaginstat (COR388) is a first-in-class, orally administered virulence factor inhibitor that targets P. gingivalis gingipains, a specific, infectious pathogen found in the brain of Alzheimer’s patients and known to cause neurodegeneration and neuroinflammation in animal models. The company’s lead investigational medicine, COR388, is currently undergoing a phase 2/3 GAIN Trial in patients with mild to moderate Alzheimer’s.
The Company was founded based on the work of Dr.Stephen Dominy, its co-founder and chief scientific officer, who discovered pathogenic bacteria in the brain of those afflicted with Alzheimer’s. This led to the development of the Company’s small molecule program aimed at preventing the progression of the disease.
Cortexyme initiated a Phase 2/3 clinical trial named (GingipAIN Inhibitor for Treatment of Alzheimer’s disease) “GAIN” in April 2019, to test the efficacy of its new investigational medicine COR388, targeting P. gingivalis bacteria. The study has enrolled 643 subjects and has passed futility analysis and will continue to 1-year endpoint based on the recommendations of the independent Data Monitoring Committee (DMC). The co-primary endpoints of the study are mean change in ADAS-Cog 11 and ADCS-ADL from baseline to 48 weeks versus placebo. The topline data from the study is expected to be available by December 2021. Furthermore, the Company is also anticipating results from a periodontal disease clinical study in Q4 2021.

Image Source: Cortexyme
The Study is based on scientific evidence that Porphyromonas gingivalis, or P. gingivalis, hitherto associated with chronic periodontal disease, can cause Alzheimer’s. COR388 has the potential to slow the progression of AD by destroying the toxic proteins, or gingipains, released by P. gingivalis. It is a first-of-its kind trial that is testing a new paradigm in the treatment of Alzheimer’s, as till date amyloid plaques were the center of any study associated with creating a solution for Alzheimer’s. It enjoys composition of matter patent protection until 2035, with more patents pending for approval.
In pre-clinical studies, COR388 has demonstrated the ability to reduce the bacterial load of a P. gingivalis infection, block the production of amyloid beta, reduce neuroinflammation, and protect neurons in the hippocampus, a part of the brain that mediates memory.

Image Source: Cortexyme
The Company announced a pipeline expansion after the successful completion of the GAIN Trial’s interim analysis. Atuzaginstat will be evaluated in a phase 2 PEAK trial for treatment of Parkinson’s disease. In addition, COR588, a novel lysine gingipain inhibitor will advance to clinical trials in Q3 2021.
Anavex Life Sciences Corp (NASDAQ: AVXL)
Market Cap: $1.76B; Current Share Price: 25.17 USD
Data by YCharts
Anavex is developing a pipeline of therapeutic candidates that target the Central Nervous System (CNS) by leveraging its expertise in precision genetic medicine. The Company’s lead candidate ANAVEX®2-73 has demonstrated improved Mini Mental State Examination (MMSE) and Alzheimer’s Disease Cooperative Study Group – Activities of Daily Living Inventory (ADCS-ADL) scores through 148 weeks in a Phase 2a clinical study. In addition, the Company has been able to identify novel genomic biomarkers such as the precision medicine biomarker, SIGMAR1 gene expression, which has led to them being applied to a Phase 2b/3 Alzheimer’s disease (AD) stud, along with other potential indications including Parkinson’s Disease Dementia (PDD) and Rett Syndrome (RTT).

Image Source: Company
In June 2021, the Company announced that it has exceeded the target of enrollment for the ANAVEX®2-73 (blarcamesine) Phase 2b/3 study in Alzheimer’s disease, the topline results from which are expected to be announced in mid-2022. The study will be using the SIGMAR1 gene expression, which has shown significant clinical benefit in cognition and activities of daily living and function, in an earlier Phase 2a Alzheimer’s disease study. The study will have 450 patients and will be held at 52 sites across North America, Europe and Australia.
ANAVEX®2-73-RS-001 is being evaluated in a Phase III clinical trial to treat pediatric patients with Rett syndrome and has shown statistically significant improvement in RSBQ (Rett Syndrome Behavior Questionnaire) scores and CGI-I scores, when compared to a placebo. The drug was found to be well -tolerated, safe and registered good patient compliance.
ANAVEX®2-73 is undergoing a phase II clinical trial for the treatment of Parkinson’s Disease Dementia (PDD) and has demonstrated significant dose dependent improvements in the quality of episodic memory. Furthermore, the Company’s pipeline consists of ANAVEX 3-71 intended for the treatment of Frontotemporal Dementia and neurodegenerative diseases; ANAVEX 1-41 for the treatment of Depression, Stroke and Neurodegenerative Diseases and ANAVEX-1066 for the treatment of Visceral Pain and Acute and Neuropathic pain.
The Company retains worldwide rights to all of its product candidates and has a strong intellectual property rights portfolio. Anavex has a cash runway of 36 months and access to non-dilutive cash resources such as the Michael J Fox Foundation, Rettsyndrome.org, Australian government.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours.
Click here to please visit our detailed disclosure
References
https://axsometherapeuticsinc.gcs-web.com/static-files/4a50801a-d8f7-489b-99dd-2d2c28828471
http://www.anavex.com/wp-content/uploads/2021/04/Anavex-Presentation-April-2021.pdf





Sorry, the comment form is closed at this time.